1. Introduction
Obesity is a chronic disease whose prevalence is increasing worldwide and it is known as a risk factor for many other chronic diseases, having been associated, by itself, with vitamin D deficiency [1].
Low levels of vitamin D observed in obeses have been linked to several factors. Some causes have been suggested such as a lesser exposure to sunlight due to decreased mobility and excess clothing, and an increase on the storage of vitamin D in adipose tissue [2]. However, overweight may also be a result of low vitamin D levels due to increased PTH levels and consequent increased influx of calcium in adipocytes, and also because this vitamin could be an inhibitor of adipogenesis [3,4]. NVDR (Nuclear Vitamin D Receptor) polymorphisms have also been associated with susceptibility to obesity in humans [5].
The objective of this study was to evaluate vitamin D status in obese patients, before and after bariatric surgery, and to examine the relationship of 25(OH)D3 levels with anthropometric measurements and body fat.
2. Methods
Retrospectively, during 5 months, data of 239 patients with measurement of 25(OH)D3 who attended the Morbid Obesity Consultation of Endocrinology Service of Hospital de São João, in Porto, were collected. 179 had undergone bariatric surgery at a mean of 20 months before, while 60 had not undergone this surgery. These data included age, gender, 25(OH)D3, anthropometric measurements and body fat.
In patients undergoing bariatric surgery, were also collected the date of surgery, and weight before surgery, and calculated weight loss and BMI reduction, as well as the time interval since surgery.
The biochemical assays were measured in the central hospital laboratory. Serum 25(OH)D3 was determined using electrochemiluminiescence immunoassay on Cobas e411 analyzer (Roche Diagnostics), with reference ranges of 30-80 ng /mL to normal values and < 15 ng / mL to vitamin D deficiency. Thus, patients were considered to have vitamin D deficiency if their serum levels of 25(OH)D3 were below 15 ng / mL.
The months of serum sample collection were grouped into winter, spring, summer or autumn. The antropometric measurements (height, weight and waist circumference (WC)) were performed by a nutritionist and according to international standards. Height and weight were measured with a stadiometer (SECA 220) and a digital scale (SECA 701), and were measured to the nearest of 0.5 cm and 0.1 Kg, respectively.
WC was measured to the nearest of 0.1 cm.
Body fat was measured by bioimpedance, with the device Omron BF-320, according to standard procedures.
Postoperatively, the patients start a daily multivitamin supplement, which contain 200 IU of vitamin D.
The project was approved by the Ethics Committee of Hospital de São João, EPE.
Statistical analysis was performed using SPSS (version 17, Chicago, USA). Data are expressed as mean ± standard deviations.
Pearson correlation was used to quantify linear associations between variables.
Stepwise multiple regression analysis was used to determine the dependence of 25(OH)D3 levels (dependent variable) over several other variables (independent variables).
A p value < 0.05 was considered statistically significant.
3. Results
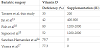
The characteristics of the study sample are shown in Table 1.
Using the criteria of 25(OH)D3 <15ng/mL, the prevalence of vitamin D deficiency was 50% in the non-operative group and 33% in the bariatric surgery group.
There was a negative association between 25(OH)D3 and anthropometric measurements in both groups. Body fat was also inversely related to 25(OH)D3, and strongly associated with 25(OH) D3 when compared with anthropometric measurements (Table 2).
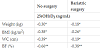
It also found a positive association between 25(OH)D3 and the amount of weight loss after bariatric surgery (Figure 1).
Stepwise backward multiple linear regression was performed to relate 25(OH)D3 (dependent variable) with independent variables: gender, age, height, weight, WC, BF, BMI, time interval since surgery, BMI reduction and season (r=0,528; p<0,001).
In this model, gender, season and BMI were predictors of 25(OH) D3.
Men have an extra 10.8 ng/mL of 25(OH)D3 over women (p = 0.001). Each BMI increase of 1 kg/m2 was associated with a decrease of 0.5 ng/mL in 25(OH)D3 (p = 0.003).
Summer and fall levels of 25(OH)D3 are 6.7 ng / dL (p = 0.010) and 5.3 ng / dL (p = 0.0024), respectively, higher than Winter.
4. Discussion
Vitamin D deficiency seems to be a prevalent problem in the obese population. In this study, a prevalence of vitamin D deficiency of 50% in the non-operative group was found. In the post-surgery group, this percentage is lower ( 33%). However, we need to consider that these patients are doing supplementation.
Other studies have reported vitamin D deficiencies in obese patients ranging from 41% to 93.8% [6,10-17]. This wide range may be partly due to the differences in definition of vitamin D deficiency (Table 3).
Goldner et al shows that the prevalence of vitamin D deficiency in pre-operative obese patients was higher than in non-obese controls (61% and 12%, respectively), even after controlling for sunlight exposure and dietary intake of calcium and vitamin D [6].
Studies in post-surgery patients have reported vitamin D deficiencies ranging from 42% to 81% [13-15,17,18]. However, there are differences in oral vitamin D supplementation (Table 4).
It was found a negative association between 25(OH)D3 and weight, BMI, WC, and body fat in both groups. These associations have been described by several authors, both in obese and non-obese patients [8,10,11,16,18-26].
In the present study, the strongest correlation was found with body fat. Snijder et al also observed that total body fat percentage was more strongly associated with 25(OH)D compared with anthropometric measures [8].
It was also found a positive association between weight loss and BMI reduction after bariatric surgery and 25(OH)D3 levels. Some studies have compared the vitamin D levels before and after bariatric surgery and assessed its relationship with weight loss [14,15,27]. Signori et al has found a higher percentage of patients with baseline vitamin D deficiency (86%) compared with the one year (post-surgical) levels (70%) [15]. Sanchez-Hernandez et al, found that postoperative calcidiol levels disclosed a significant inverse relationship with weight loss [14]. Shahar et al also observed an increase in serum 25(OH) D among participants who lost more weight, despite the natural decrease in sun exposure from baseline (summer) to the 6 month visit (winter) [26].
BMI, beyond gender and season, is also an independent predictor of 25(OH)D3. A BMI increase of 1 kg/m2 was associated with a decrease of 0.5 ng/mL in 25(OH)D3.
Other authors had also found this association. Stein et al found that each 1 kg/m2 increase in BMI was associated with a 1.3 nmol/l decrease in 25(OH)D (P < 0.01) [16]. Other authors showed a decrease of 0.74 nmol/l (p = 0.002) in vitamin D3 per 1 kg/m2 increase in BMI 25.
There are several limitations in this study. Information on sun exposure and on vitamin D intake was not available, as well as the initial levels of 25(OH)D3 in postoperative patients and the measurements of PTH. In addition, there was a lack of a non-obese control group.
In conclusion, the levels of vitamin D seem to be associated with obesity. This finding suggests the need for assessment of vitamin D status and oral vitamin D supplementation in this population. Further studies are required to examine the causal association between serum vitamin D levels and obesity.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
A. Tavares, BMPM. Oliveira, A. Varela, P. Freitas, António Raposo and Flora Correia, made an active contribution to the conception and design of the present work, as well as a deep analysis and interpretation of the data. This paper was also drafted and its content was critically reviewed by all the co-authors: A. Tavares, BMPM. Oliveira, A. Varela, P. Freitas, António Raposo and Flora Correia, which approved the final version submitted for publication.
Abbreviations
25(OH)D: 25-hydroxy vitamin D,
PTH: Parathyroid hormone,
NVDR: Nuclear Vitamin D Receptor,
BMI: body mass index,
WC: Waist circumference,
BF: Body fat