1. Introduction
In order to archive skin lightening and depigmentation, whitening relative products have been attention in cosmetic industrial. Among of commercial skin-whitening chemicals, L-ascorbic acid relative derivatives are reliable and evidence since simultaneously provided whitening, anti-oxidation potential and prevented the formation of wrinkles [1]. The past research also demonstrated that L-ascorbic acid relative derivatives have more benefits not only at skin surface to photoprotection from ultraviolet A and B, but also influence in dermis to promote collagen synthesis [2]. Because of the unstable oxidative property of L-ascorbic acid, modification of its structure and synthesis of derivatives are effective strategies to improve the degradation [3].
Utilizing ion salts (magnesium or sodium salts) to bind molecule entity of L-ascorbic acid to form a complex, such as magnesium L-ascorbyl phosphate (MAP) and sodium ascorbyl phosphate which are more stable alternatives to L-ascorbic acid. The ion salts derivatives have been used in commercial products, which provided whitening potential, protecting UVB induced skin damage and stimulating collagen synthesis ability [4]. Even though MAP has been proved more stable than L-ascorbic acid, it still degrades slowly when exposed to light and air. Subsequently, L-ascorbic acid 2- glucoside (AA2G) has been designed to avoid its early degradation.
The hydroxyl group of the second carbon (C2) of L-ascorbic acid is the predominant site, which influences the pharmacologic activity; however, C2 site also controlled the degradation rate. Hence, the use of structure-activity relationships (SAR) concept to modify L-ascorbic acid, which is conjugated with glucose to the hydroxyl group of the C2 by an α-1, 2-linkage to L-ascorbic acid [5]. The results have been evidences that AA2G could facilitate the stability including protected degradation from high temperatures, pH and metal ions. Generally, AA2G are hydrolysed by α-glucosidase in which can be found in the skin cells, and converted into free L-ascorbic acid [6]. Hence, all the biologic activities of L-ascorbic acid were remained.
Topical delivery is a direct delivery route for cosmetic or pharmaceutical administration. Actually, the skin is a heterogeneous membrane; lipophilic on its surface and hydrophilic in its deeper layers. The stratum corneum is the outermost part of the skin with a highly resistant barrier which limits the penetration of drugs into the skin because its structure contributes to its function both as a barrier to water loss and as a barrier against the external environment [7]. The stratum corneum is therefore the main barrier in topical or transdermal delivery process [8]. Because of its hydrophilic character, AA2G has lower ability to pass through to stratum corneum and penetrate into the skin.
In order to facilitate the penetration of AA2G and its relative derivatives to across skin, previous literatures utilized many sophisticated formulation, such as liposomes, nanoparticles or micro-emulsions [9]. In the cosmetic manufacturing industrial field, easy preparing process and stability product is the major concern. There have been widely used in various cosmetic products including moisturizing and soothing agents, body cleansing agents, hair care compositions, antiperspirants, sunscreens and perfume. Particularly in the high hydration properties which make the skin rapid cutaneous penetrated into the skin [10]. A microemulsions therefore is the best choice due to the properties of spontaneously formation, do not by using any mechanical forces. The key factor of a successfully formulation is based on suitable composition and its ratio [11]. Based on the above, preparing and formulating of cosmetic product is a routine process in the cosmetic manufacturing, it should be maintain non-reducibility and stable of its own active ability at process and storage conditions. Actually, incorporation with AA2G product which would be accepted rang of pH is just between 5.5 and 7.0 12. Moreover, it could be heated to 50°C maintain for 20 days. However, the hydrophilicity of AA2G limits the penetration ability. The aim of the study was to optimized microemulsion formulation which easily preparation and stabilized in the cosmetic industrial and enhance the penetration ability into skin simultaneous remain lightening effect.
2. Materials And Methods
2.1 Materials
AA2G was obtained from Kalin Enterprise Co, Ltd (Taipei, Taiwan). Isopropyl myristate (IPM), Span20 and Tween80 were purchased from Sigma-Aldrich (St Louis, MO). Other chemicals used in the study were reagent grade.
2.2 Preparation of AA2G microemulsion
The oil and aqueous phase were separately prepared. Two kinds of mixture of surfactant: Tween 80/Span 20 = 3:2 were used. The oil phase consisted of IPM and mixture surfactants with the weight ratio were 2:3, total consisted 1g, while the aqueous phase consisted of double-distilled water 2% AA2G. The aqueous phase about 25μl was added to the oily phase and shaken by a vortex for 2min at room temperature. The clear o/w microemulsions were obtained.
2.3 Microemulsion characterization
Vehicle size was measured by laser light scattering with a heliumneon laser at 630 nm (Zetasizer 3000HSA, Malvern, Worchestershire, UK) matained at 25°C. The polydispersity index (PI) was used to measure size distribution. All vesicles were diluted 80-fold with deionized water before size measurements were taken. The determination was repeated three times per sample for three samples.
2.4 Morphological observations by transmission electron microscopy (TEM)
AA2G microemulsion was examined by TEM to characterize their microstructure. A drop of samples was applied to a carbon film covered copper grid to form a thin-film specimen (Ted Pella Inc, Redding, CA). The sample was then examined and photographed with a Jeol JEM-1230 TEM (Tokyo, Japan) at an acceleration voltage of 80 kV.
2.5 AA2G analysis by HPLC
AA2G was analyzed with an HPLC system consisting of a Hitachi L-7100 pump, a Hitachi L-7200 sample processor, and a Hitachi L-7400 ultraviolet detector. A25-cm-long, 4-mm-inner-diameter stainless-steel RP-18 column 5 μm (Merck, Darmstadt, Germany) was used. The mobile phase was a mixture consisted of 0.7 g of potassium dihydrogen phosphate dissolved in 1,000 mL of double-distilled water adjusted to pH 3 with 0.1M citric acid at a flow rate of 1 ml/min. The wavelength of ultraviolet detector was set at 254 nm.
2.6 In vitro release and skin permeation ability
AA2G release rate and skin permeation ability from the microemulsion was measured with a modified Franz vertical diffusion assembly (Ching Fa, Hsinchu, Taiwan). Cellulose dialysis membranes (CellSep®T2, MW cut-off of 6000-8000, Membrane Filtration Products, USA) with a molecular weight cutoff of 6000-8000 Da were soaked in double-distilled water for 12 h before the experiment. Then, either a membrane or normal nude mouse skin was mounted between the donor and receptor compartments as the barrier. The donor medium consisted of 0.5 ml of a control solution or microemulsion formulation. The receptor medium consisted of 5.5 ml of pH 7.4 citrate-phosphate buffer. The available diffusion area was 0.785 cm2. The stirring rate of the receptor was 600 rpm, and the temperature was maintained at 37°C. At appropriate intervals at 1, 2, 4, 6, 8, 10, 12 h, 200 μl aliquots of the receptor medium were withdrawn and immediately replaced with an equal volume of fresh buffer. The release accumulative amount of AA2G was determined by HPLC.
2.7 Skin deposition
The amount of AA2G retained in the skin was determined at the end of the in vitro experiment (12 h). The application site on the skin was washed 10 times using a cotton cloth immersed in water. A sample of skin was weighed on an analytical balance, cut with scissors, homogenization with 0.5 mL of methanol for 3 min. The resulting solution was centrifuged for 10 min at 12,000 rpm. The supernatant was filtered across a 0.45-mm PVDF membrane and then analyzed by HPLC.
2.8 Skin irritation and whitening evaluation by colormerty
The experiment protocol was reviewed and approved by the institutional review board (IRB) ethics committees of Yuanpei University, Hsinchu, Taiwan. The volunteers of same sexes were of age group between 20-30 years. The healthy human volunteers not suffered from any skin disease, the health examination criteria of each volunteers. All volunteers were informed that do not wash the treatment area during experiment period. The skin irritation from AA2G microemulsion was applied on the forearm of 8 healthy volunteers. The evaluation of skin irritation level was performed by using colorimetry (Chroma Meter-CR 221, Minolta, Japan). AA2G microemulsion about 500 μl was uniformly spread applied to the inner area of arm. The polyethylene cloth was fixed with Tegaderm® adhesive dressing (3M, MN, USA). After 24 h, the cloth was removed, and the treated skin area was swabbed clean with a cotton wool swab. Thirty minutes after withdrawing the vehicle, the colorimetry of the treated skin was measured. The skin irritation level in terms of erythema color was measured by colorimetry (Chroma Meter- CR 221, Minolta, Japan). The instrument records three-dimensional color reflectance (including L*, a*, and b*), as recommended by the Commission International del’Eclairage (CIE). The definitions are as follows: L* is the relative brightness ranging from total black (0) to total white (+100), a* is the balance between red (+100) and green (-100), and b* is the balance between yellow (+100) and blue (-100). The total difference in color between the light-treated site and untreated site as the control is defined as follows:
Δc = (Δa2 + Δb2)1/2 Δc = (ΔL2 + Δa2 + Δb2)1/2
2.9 Statistical analysis
All data reported are expressed as the mean ± standard derivation. Statistical analyses were performed using an unpaired Student’s t-test with Winks SDA 6.0 software (Texasoft, Duncanville, TX). An analysis of variance test was also used. Subgroup comparisons were made using the Newman-Keuls multiple comparisons. A 0.05 level of probability was used as the level of significance.
3. Results and Discussion
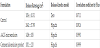
AA2G™ was originally developed as a quasi-drug (functional cosmetic products) in Japan to lighten the overall tone of the skin and reduce the pigmentation in age spots and freckles. Past research sophisticated has shown other dramatic benefits and today AA2G™ is used all over the world – not only for whitening but also for brightening dull looking skin, reversing the effects of aging, and in sunscreen products for protection. However, the use of AA2G in cosmetic products has been limited owing to its labile oxidative properties, which can be accelerated in the presence of oxygen, water, light, heat and metals [13,14]. The presented study demonstrated that utilizing micromulsion formulation carry AA2G would be archive the better permeation and provide whitening beneficial
3.1 The preparation and properties of AA2G microemulsion
In order to easy preparation the formulation in the large scale manufacture process, microemulsions were selected in this study. The correct ingredient and percentage would successful to prepare the microemulsions. The area of microemulsion isotropic region in the presence of cosurfactant (ethanol) was comparably larger than in the absence of cosurfactant. This is consistent with previous studies [15,16], which reported that cosurfactant acting predominantly in the aqueous phase is generally more effective at producing microemulsions over a wide range of compositions than those mainly acting in the interfacial film region. The phenomenon may be due to the fact that the cosurfactant could decrease the hydrophilicity of the polar solvent; hence the water-in-oil microemulsions were capable of solubilizing high water content [17]. Our past experience also indicated that the clear and low viscosity microemulsion relative with HLB of incorporating surfactant. Moreover, cosurfactnat also play a predominantly effect. When incorporating with ethanol as cosurfactant, the thermodynamic activity would changes in current colloid system. The comparison of microemulsion and emulsion of particle size are shown in Figure 1. We measured particle size of microemulsion with or without AA2G, and commercial emulsion product which presented 143, 186 and 1844 nm, separately. Moreover, the particle size of AA2G microemulsion were distributed from 50- 100, 100-150, 150-200 nm presented 19.2, 31.7 and 45.6 % of intensity. In terms of appearance, microemulsion formulations containing 2% AA2G in in this study were clear and transparent solutions; no precipitate was observed. Figure 2 shows the morphology of AA2G microemulsion on TEM. The images reveal that the microemulsion droplets had nanometer-sized spherical shapes and a well-dispersed pattern. Figure 3 presents a schematic depiction of the AA2G microemulsion system.
3.2 In vitro release study
In vitro release study of AA2G from microemulsion and commercial emulsion were measured as the cumulative amounts in the receptor compartment, and calculated the parameters of the release kinetics from the three kinds of nanoparticles to explore the release behavior. As shown in Figure 3, the release rate of the different types of formulation decreased in the order of control > microemulsion > commercial emulsion. The control group showed more-rapid release behavior when compared to the microemulsion or emulsion.
Table 1 summarized the accumulative amounts and release rates of AA2G from microemulsion and emulsion carrier. Despite of different release rate, control group, microemulsion and commercial emulsion were presented hiquchi model. According to the release rate, the results indicated that AA2G control solution released in pH medium was fast, and commercial emulsion product incorporated much composition which may obstructed release rate. The phenomenon contributed thermodynamic activity, which made the colloid system changes by the excipients.
3.3 Skin penetration ability and skin deposition
AA2G was hydrophilicity which presented lower penetration ability also oxidation in the preparation process. Therefore, to evaluate the skin penetration amount is more important. The skin penetration ability of microemulsion, we utilized in vitro Franze diffusion model across to nude mouse skin to screen the formulation. As shown in Figure 4, it was found that the control group (AA2G dissolved in H2O) presented a weak signal from HPLC. Previous studies also presented the lower permeated ability [18,19]. Moreover, cumulative amounts of AA2G from microemulsion were significantly higher than the commercial emulsion product (p<0.05). By contrast, the result of AA2G deposition within skin was as the same as the cumulative amounts results, as shown in Figure 4. Table 2 summarized the Cumulative amounts, penetration model, flux and enhancement ratio. Overall, the penetration ability and skin deposition of AA2G was enhanced by microemulsion rather than commercial emulsion. The enhacement ratio of AA2G microemulsion was higher than control group about 100 fold. Although commercial emulsion product was increased in the enhancement ratio, however only 18 fold. Additionally, comparison with release behavior, AA2G control almost not measured in the both buffer medium and skin deposition. The phenomena indicated that the hydrophilic property of AA2G not penetrated across to the skin or retained in the skin despite the small molecular. Moreover, the penetration ability of commercial emulsion product slight higher than AA2G control. The skin penetration ability may limit by the micron scale particle size and the multiple ingredient. The stratum corneum is the main obstruct limited the hydrophilic ingredients pass through skin [20,21].
3.5 Colormetry assessment in human study
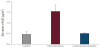
The study was performed volunteer female to evaluate the skin irritation and whitening effect by colormetry. For the colorimetric evaluation, the values of a* was used as the balance between red and green, which indicated the irritation indicator. Δa* was compared to control (untreated skin), after exposure AA2G microemulsion to skin for 1, 24, 48 and 72 h the data revealed that the indicators did not significantly change, as shown in Figure 5. The positive results indicated that AA2G microemulsion had no irritation effect. Moreover, lightening and colorful of skin was also evaluated by colormetry. The differences in colors between the control and AA2G microemulsiontreated groups were expressed as lightening (Δc*) and colorful (Δe*), which had a value of +4.232 and +3.9926, respectively. AA2G is well known as the whitening ingredient which required give a suitable formulation to facilitate the permeation into skin to archive the whitening function.
4. Conclusion
In summary, microemulsion formulations containing AA2G were successful characterized in this study. AA2G microemulsion was prepared an average diameter approximately 150 nm. Skin penetration and skin deposition of AA2G microemulsion was higher than both control aqueous solution and commercial emulsion product. The positive results demonstrated that AA2G microemulsion is potential penetration carrier in topical usage. Moreover, in vivo human study demonstrated the AA2G microemulsion had better whitening effect and did not cause irritation.
Competing Interests
The authors declare that they have no competing interests.
References
- Raschke T, Koop U, Düsing HJ, Filbry A, Sauermann K, et al. (2004) Topical activity of ascorbic acid: from in vitro optimization to in vivo efficacy. Skin Pharmacol Physiol17: 200-206. View
- Farris PK (2005) Topical vitamin C: a useful agent for treating photoaging and other dermatologic conditions. Dermatol Surgery 31: 814-817. View
- Balaguer A, Chisvert A, Salvador A (2008) Environmentally friendly LC for the simultaneous determination of ascorbic acid and its derivatives in skinwhitening cosmetics. J Sep Sci 31: 229-236. View
- Geesin JC, Gordon JS, Berg RA (1993) Regulation of collagen synthesis in human dermal fibroblasts by the sodium and magnesium salts of ascorbyl- 2-phosphate. Skin Pharmacol 6: 65-71. View
- Nakamura S, Oku T (2009) Bioavailability of 2-O-alpha-D-glucopyranosyl- L-ascorbic acid as ascorbic acid in healthy humans. Nutrition 25: 686-691. View
- Spiclin P, Homar M, Zupancic-Valant A, Gasperlin M (2003) Sodium ascorbyl phosphate in topical microemulsions. Int J Pharm 256: 65-73. View
- Pai VV, Bhandari P, Shukla P (2016) Topical peptides as cosmeceuticals. Indian J Dermatol Venereol Leprol . View
- Förster M, Bolzinger MA, Fessi H, Briançon S (2009) Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur J Dermatol 19: 309-323. View
- Moribe K, Limwikrant W, Higashi K, Yamamoto K (2011) Drug nanoparticle formulation using ascorbic Acid derivatives. J Drug Deliv 2011: 138929. View
- Azeem A, Rizwan M, Ahmad FJ, Khan Z, Khar RK, et al. (2008) Emerging role of microemulsions in cosmetics. Recent Pat Drug Deliv Formul 2: 275- 289. View
- Lu GW, Gao P (2010) Emulsions and microemulsions for topical and transdermal drug delivery. Handbook of Non-Invasive Drug Delivery Systems, pp. 59-94. View
- Inoue Y, Yoshimura S, Tozuka Y, Moribe K, Kumamoto T, et al. (2007) Application of ascorbic acid 2-glucoside as a solubilizing agent for clarithromycin: Solubilization and nanoparticle formation. Int J Pharm 331: 38-45. View
- Campos PM, Gonçalves GM, Gaspar LR (2008) In vitro antioxidant activity and in vivo efficacy of topical formulations containing vitamin C and its derivatives studied by non-invasive methods. Skin Res Technol 14: 376- 380. View
- Raschke T, Koop U, Düsing HJ, Filbry A, Sauermann K, et al. (2004) Topical activity of ascorbic acid: from in vitro optimization to in vivo efficacy. Skin Pharmacol Physiol 17: 200-206. View
- Wu PC, Lin YH, Chang JS, Huang YB, Tsai YH (2010) The effect of component of microemulsion for transdermal delivery of nicardipine hydrochloride. Drug Dev Ind Pharm 36: 1398-1403. View
- Szumala P (2001) Structure of Microemulsion Formulated with Monoacylglycerols in the Presence of Polyols and Ethanol. Journal of surfactants and detergents 18: 97-106. View
- Bagwe RP, Kanicky JR, Palla BJ, Patanjali PK, Shah DO (2001) Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit Rev Ther Drug Carrier Syst 18: 77-140. View
- Hsiao CY, Huang CH, Hu S, Ko YS, Sung HC, et al. (2012) Fractional carbon dioxide laser treatment to enhance skin permeation of ascorbic acid 2-glucoside with minimal skin disruption. Dermatol Surg 38:1284-1293. View
- Hikima T, Tamura Y, Yamawaki Y, Yamamoto M, Tojo K (2013) Skin accumulation and penetration of a hydrophilic compound by a novel gemini surfactant, sodium dilauramidoglutamide lysine. Int J Pharm 443: 288-292. View
- Andrews SN, Jeong E, Prausnitz MR (2013) Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm Res 30: 1099-1109. View
- Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26: 1261-1268. View