1. Introduction
In response to the demands of various industries for light structural materials, different aluminum lithium alloys have been developed [1]. These alloys are commonly selected because they exhibit an enhanced strength/weight ratio and therefore are typically applied as functional materials with high performance in the aerospace and automobiles industries. Economically, Al-Li alloys are only three times as expensive as conventional aluminum alloys, whereas competing hybrid materials can be up to 10-30 times more expensive [2]. In the context of high fuel prices and fierce competition between composite, hybrid materials and aluminum alloys, different Al-Li-Cu alloys have been developed to overcome most of technical issues associated with the formerly available materials [3]. In the space industry, for instance, Al – Li – Cu based alloys are the candidate material for the construction of launchvehicle liquid-propellant tanks because the ductility and toughness of the alloy are not affected at the lower temperatures. These alloys are known to be strengthened through precipitation hardening and are attracting strong interest in the framework of the development of new alloys, particularly for aerospace components [4]. The current study selects one of the widely used Al-Li-Cu alloys from the Weldalite family [1], namely, the commercial AA2195. This alloy was developed to replace the AA2219 alloy, which was conventionally used in the US space shuttles [5]. The AA2195 is a weldable alloy of high strength that provided a considerable mass reduction and increase in the payload capacity of the space shuttle [5]. This class of alloy is able to achieve superior mechanical properties in the stretched and artificially aged condition (termed as T8), artificially aged condition (termed as T6) and naturally aged condition (termed as T4) [6].
Previous studies revealed that the Al alloys in T4 temper leads to strengthening by a combination of the G.P zone and δ'(Al3Li) precipitates [7]. The alloy in this temper displays a rapid and strong naturally aging response, even without prior cold working. For example, the tensile strength of Weldalite grade alloy in the T4 temper is 20% greater than that of the leading, weldable alloy AA2219 in the T87 temper [8].
The Al alloys with the T6 temper are reported to be strengthened primarily by the T1 (Al2LiCu) phase, with a minor presence of the θ' (Al2Cu) phase [9]. The alloy in this temper exhibited resistance to corrosion during the retrogressed and raged process [10]. This corrosion resistance can be explained in the terms of the coarsening of the grain boundary precipitates. In addition, the increase in the volume fractions of the second phase particles at the grain interiors was responsible for the increase in the strength of the alloy following this temper [11]. In addition, a two-step T6 aging treatment was demonstrated to result in strength and ductility comparable to those of samples in the T8 temper [10].
For the Al alloy in the T8 condition, the microstructure, as revealed by transmission electron microscopy, mainly consisted of the δ' (Al3Li) and T1 (Al2LiCu) precipitates as the primary strengthening phases [12]. The application of plastic deformation prior to artificial aging is used to promote a uniform distribution of heterogeneously nucleated T1 precipitates. The distribution of the T1 precipitates in the microstructure after conducting the T8 temper is considered to be responsible for the improvements in the strength and fracturetoughness. The plastic deformation can be effective for strengthening aluminum alloys processed at cryogenic and room temperatures.
In the quenched state and the early stage of aging, the microstructure shows a mixture of well-defined ordered δ' (Al3Li) and complex δ' (Al3Li) / β'(Al3Zr) ordered precipitates with spherical and polygonal shapes in the Zr containing alloy [13]. After solution aging treatment, most of the Zr are found within small spherical (Al3Zr) dispersoids that are approximately 20 nm in diameter [14].
The objective of this study is to investigate the dependence of the precipitation kinetics and the phase transformations in complex Al- Li alloys on the preformed heat treatment conditions. AA2195, a commonly used aluminum alloy, was selected as a model system. The heat treatments considered in this study were common industrial heat treatments: T8 temper (solution treated, cold working and artificially aging), T4 temper (solution treated and naturally aging) and T6 temper (solution treated and artificially aging) [3]. The use of a combination of experimental methods – a laser-assisted wide angle tomographic atom probe (LAWATAP), field ion microscopy (FIM), transmission electron microscopy (TEM), differential scanning calorimetry (DSC) and Vickers micro hardness measurements – allowed for a qualitative evaluation of the precipitation kinetics and phase transformation as functions of the temper conditions.
2. Experimental
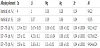
Plates of 1-mm thick alloy AA2195 were subjected to the following treatment conditions: 1) T8 (solution treatment for 1 h at 510°C, water quenching, plastic deformation of 3% and artificial aging for 30 h at 150°C), 2) T4 (solution treatment for 1 h at 530°C, water quenching and natural aging at room temperature for 4000 h), and 3) T6 (solution treatment for 2 h at 495°C and for 4 h at 515°C, water quenching and artificial aging for 20 h at 180°C). The chemical compositions of the samples following treatment under the T8, T4 and T6 conditions are presented in Table 1. The measured compositions using inductively coupled plasma optical emission spectrometry using an ICPOEC 720-ESinstrument are in a good agreement with the nominal composition in all cases.
The influence of different heat treatment conditions on the mechanical properties was studied using the Vickers hardness test. The samples were polished by using standard metallographic processes (grinding and polishing by using colloidal silica). Vickers micro-harness measurements were performed using a load of 0.2 kg applied on the samples for 10 seconds. The hardness values were determined by an average of 10 measurements.
DSC was performed at a heating rate of 10 K/min in a Netzsch DSC 204 F1 aperture. The mass specimen between 12 and 16 mg was placed in an Al crucible in a dynamic nitrogen atmosphere (20 ml/ min). The thermal properties of AA2195 for different tempers were examined.
TEM analysis was performed after each specific treatment. Electrontransparent foils were grinded to an approximate thickness of 150 μm, mechanically punched, and then electro-polished by standard twinjet polishing using a solution of 20 vol % of nitric acid in methanol at -20°C. The analysis was performed using a JEOL 2100F apparatus operated at 200 kV.
Microstructural characterization at the atomic scale was performed using atom probe tomography (APT). Atom probe studies were performed using both LAWATAP in the voltage mode at 30 K with a pulse fraction of 20 % and at a vacuum level of 10-8 Pa. Small rods of (0.3 mm × 0.3 mm × 10 mm) were spark machined from the bulk and then electro-polished to a sharp needle shaped specimen for APT analysis and FIM observation. Electro-polishing was performed using a solution of 30 vol % of nitric acid in methanol at – 20 °C in the range of 5 to 7 V. FIM images were obtained on LAWATP using Ne as the imaging gas at 25 K and 1.2 x 10-3 Pa.
The obtained APT data were visualized using the TAP3D and IVAS software programs provided by Cameca. Calibration of the main reconstruction parameters was performed by observing the atomic plane for the respective crystallographic pole. The composition of the different observed precipitates was measured quantitatively using the proximity histogram (prixogram) technique [17]. In this methodology, an isoconcentration surface first delineates the precipitates. Next, starting at the isoconcentration surface, which is the origin of the proxigram, and then moving toward the center of precipitates (positive distance), the composition of the precipitate is measured according to discrete shells with the shape of isoconcentration surface at fixed intervals. The proxigrams in this study were selected with a spacing of 0.1 nm between these shells. The matrix composition is obtained by starting at the isoconcentration surface and moving into the matrix (negative distance).
3. Results and Discussions
3.1 Hardness curves
Figure 1 shows the hardness evolution during the heat treatment of the alloy for different temper conditions: T4, T8 and T6. The first feature of interest is that the specimen in T8 temper has a higher hardness ((160.3 ± 7) HV/1.96) than those of the specimens in the T4 and T6 tempers. During T4 and T6 tempering, both specimens experience a significant and quite similar hardening response. The minimum hardness value is reached in T6 tempering ((112 ± 5) HV/1.96). During the natural aging of the sample in the T4 temper, no significant change of hardness is observed ((125.2 ± 9) HV/1.96). This behavior can be attributed to the presence of different nano-scale structural features after the conducting of each heat treatment temper. The explanation of most of these features from the microstructure evaluation will be presented in the following section.
3.2 TEM analyses
TEM analysis of the specimens conducting different temper conditions is shown in Figure 2. The microstructure of a naturally aged sample after conducting the T4 heat treatment is shown in Figure 2a.Precipitates were characterized using an exact [110] zone. The microstructure encompasses spherical precipitates with L12 structure. A schematic of the indexed selected area diffraction pattern (SADP) in the [101] direction is integrated with the bright field TEM image (Figure 2a). This observation indicates the presence of the supper lattice reflection, which originates from an L12 ordered structure representing β' (Al3Zr) precipitates. The L12 unit cell is based on the face center cubic (fcc) structure of aluminum. However, the corner atoms are replaced by Zr atoms. This substitution means that all <100> directions are super structure directions. The spherical precipitates are assigned to be of the β'-type and not of the δ'-type because the Li composition in the investigated alloy is less than 5 at.% (Table 1). Hence, there is no expectation to observe δ'(Al3Li) precipitates. However, the contrast of the spherical particles indicates that there are wings of low intensity surrounding the β' particles. This observation. could be due to the role of the β' phase on providing heterogeneousnucleation sites for the δ' phase or/and T1 phase [18,19].
Figure 2b shows a TEM bright field image of the specimen conducting T8 heat treatment condition. The image revealed a complex microstructure, including different precipitates with platelets and spherical morphology. Once again, precipitates were characterized using an exact [110] zone. A schematic of the indexed selected area diffraction pattern in the [101] direction in Figure 2b indicates the presence of two variants of the T1 precipitates as streaks along the <111> directions in the pattern. The other two variants of this phase produce spots adjacent to the <200> positions. The streaks along the <200> directions reveal the presence of the θ' precipitates. The super lattice reflection in the pattern is attributed to the β' precipitates. These observations provide evidence that the peak hardness microstructure consisted of the thin T1 platelets associated with the smaller fraction of Cu-rich phase, i.e., θ' platelets.
A TEM bright field image of the specimen after the T6 heat treatment is shown in Figure 2c. In this case, the matrix contained the T1 precipitates as the primary strengthening phase. It is difficult to observe any streaks along <200> directions, due to a negligible presence of the θ' precipitates in the microstructure for the specimen under the T6 condition. However, it is clear from Figure 2c that under T6 heat treatment the T1 precipitates remain quite thin and in a good dispersion.
3.3 DSC analysis
Further confirmation of the precipitation kinetics during different temper treatments was obtained by performing DSC scans for the specimens under the T4, T8 and T6 conditions. The results of the thermal characterizations are shown in Figure 3, which presents the DSC traces of the specimens in all three temper treatments. The precipitate and dissolution reactions important for thermal stability and precipitation occur in the aluminum alloy in the range of temperature of 25- 350°C [12].
The major thermal events for the specimens in the T8 condition are the endothermic dissolution peak spanning the range of ~ 180 – 220°C and the exothermic precipitation peak spanning the range of ~ 220 – 280°C. For the specimen in the T4 condition, the DSC signal shows the presence of two endothermic dissolution peaks: the first peak spanning the range of ~ 90 – 130 °C and the second spanning the range of ~ 190 – 230°C. Moreover, the presence of an exothermic precipitation peak spanning the range of ~ 230 – 310°C and an endothermic dissolution peak spanning the range of ~ 320 – 500°C are observed. Finally, the signal for the specimen in the T6 condition shows an endothermic dissolution peak at elevated temperature spanning the range of ~ 320 – 500°C.
Based on previous observations in the literature [12] and on the TEM analyses above, the peaks can be identified as the following. The endothermic dissolution peak spanning the range of ~ 90 – 130°C in the T4 condition is the result of dissolution of the GP zone (precursor of the θ' phase). The second endothermic dissolution peak spanning the range of ~ 190 – 230°C is due to the dissolution of the T1 phase. The exothermic precipitation peak spanning the range of ~ 230 – 310°C is due to the precipitation of the θ' phase. Finally, the lastendothermic dissolution peak spanning the range of ~ 350 – 500°C corresponds to the dissolution of the existing bulk phases [20].
For the T8 condition, the endothermic dissolution peak spanning the range of ~ 180 – 220°C is assigned to the dissolution of the T1 phase. However, the exothermic precipitation peak spanning the range of ~ 220 – 280°C is assigned to the re-precipitation of the T1 phase again. The re-precipitation phenomena under the dynamic heating condition in DSC have been previously reported for underaged Al-Li alloys [21]; the authors reported the retrogression of the δ^' phase, which formed during the aging process, and the subsequent exothermic peak, which corresponds to the re-precipitation of the δ' phase, were explained in terms of the increase in the number of the solute atoms in super saturated solid solution, which causes the reprecipitation to occur. In our case, the strong re-precipitation of the T1 phase observed for the specimen in T8 condition suggests that the T8 temper condition still exhibits an appreciable super saturation with respect to Cu and Li atoms. During the DSC scan with a heating rate of 10 K/min, this super saturation leads to the precipitation of an additional amount of the T1 phase [20].
Finally, the endothermic dissolution peak spanning the range of ~ 320 – 500°C in the T6 condition most probably originates from on going T1 dissolution, which is considered to be the dominant phase in the microstructure, according to the TEM image (Figure 2c).
The variations and the similarities of the thermogram curves in Figure 3 reveal the changes of precipitation processes to some extent. It is worth to note that the identification of the precipitation kinetics and phase transformations has not been conclusively established by the DSC signals. Therefore, obtaining a clear picture of the precipitation kinetics and phase transformations from the naturally aged condition (T4) to the peak-aged state (T8) and then to the slightly over-aged state (T6) required a deeper insight into the microstructure-properties relationship at the nano-scale using a global characterization technique, such as APT.
3.4 FIM observation
Prior to performing the APT analysis, the FIM mode in the LAWATAP was used. FIM was mainly used to develop the needle shaped samples for the subsequent analysis by APT. Surface oxide and damage induced by using the standard electro-polishing method could thus be eliminated. In this method, Ne gas atoms are field ionized due to the presence of a high electric field at the tip surface. The ions of the image gas are then projected onto the imaging screen, where they form a highly magnified image of the surface [22].
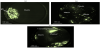
Figure 4 shows the field ion micrographs for the alloy AA2195 after conducting the three industrial heat treatments, i.e., the T4, T8 and T6 conditions. Figure 4a is the field ion micrograph for the sample undergoing the T4 heat treatment condition. This Figure revealed the presence of the spherical precipitates, which appear bright in the images. These spherical precipitates are most probably of the β'-type, as discussed above. Figure 4a, also clearly shows that the contrast within the matrix is very poor and that the Zr-rich precipitates appear very bright with respect to the dim Al matrix. This observation is due to the higher evaporation field of Zr (56 V/nm) compared to that of Al (19 V/nm) [23]. The difference on the microstructure after undergoing the T8 heat treatment condition is shown in Figure 4b. The presence of platelet shaped precipitates distributed within the matrix around the crystallographic pole that appear bright in the image is revealed. The microstructure of the sample under the T6 heat treatment condition is shown in Figure 4c. Once again, the sample consisted of platelet shaped precipitates. However, some of these platelets exhibit a slight coarsening behavior.
It is notable that all of the precipitates in this study are brightly imaged using the FIM mode. This observation can be explained by considering the field strengths of the existing precipitates, which contain Cu, Li and Zr, which are higher than that of the matrix, which is Al enriched. Accordingly, it is very likely in the FIM images that these precipitation regions protrude over the Al matrix and thus act as potential sites for imaging ions. This effect, in turn, causes the bright appearance of the precipitates in the micrographs [23].
3.5 APT analysis
The ternary Al-Li-Cu system has a complex precipitation sequence, exhibiting aspects of both binary Al-Cu and Al-Li systems. This precipitation is further complicated by the addition of minor alloying elements, such as Mg, Ag and Zr. The aging of this complex alloy generates a number of different phases. A detailed and robust investigation of the precipitation kinetics and complex phase transformations are discussed below.
3.6 Naturally aged samples for the T4 temper
Figure 5 shows the reconstructed volume of the sample in the T4 condition analyzed using LAWATAP. Based on the TEM and FIM observations above, the microstructure in this condition is expected to have only particles of β'(Al3Zr). Due to the fact of the large diameter (~ 20 nm) [14] and low number density of the β' phase in the microstructure, the APT analysis of such a phase is challenging. However, repeating the experimental analyses for the specimens in this condition permits the collection of two datasets, from which a statistically significant conclusion regarding this phase and its interface with the matrix are drawn.
Estimation of the β' precipitates was performed using a cluster identification algorithm [24]. Zr containing precipitates were identified by applying a maximum separation distance of 0.7 nm between the Zr atoms, with a minimum of 20 Zr atoms in each precipitates. Exploration of the distribution of the β' precipitates isshown in Figure 5a. The precipitates exhibit a somewhat spherical shape, with an average diameter of (10 ± 2) nm. The precipitates are then delineated by 5 at.% Zr isoconcentration surface. A proxigram composition profile from this isoconcentration surface gives the chemical composition of the β' phase as (23± 2) at.% Zr, (1.8 ± 0.5) at.% Li, (1.6 ± 0.4) at.% Cu (0.25 ± 0.1) at.% Mg and (0.4 ± 0.2) at.% Ag with an Al / Zr ratio close to 3:1 (Figure 5b). This result, also in a good agreement with the results reported by Sha and Cerezo [25], indicates precipitation of β' (Al3Zr).
The presence of β' particles in the microstructure of the naturally aged sample can be interpreted as a result of the formation of β' dispersoids from the super saturations. These β' dispersoids are very stable due to the low Zr solubility in the Al matrix, i.e., a small misfit and sluggish diffusion of Zr [15]. Zr is considered to be an important trace alloying element in a high strength Al alloy. Zr is often added to optimize the properties of the alloy and has different effects, such asrefining the grain size, inhibiting the recrystallization and improving the corrosion cracking resistance [26] and quench sensitivity of the alloy [21]. To date, the ability of the main alloying elements, such as Li, Cu, Mg and Ag, to be dissolved into these particles remains unclear [25]. The alloying elements of Zr, Li, Cu, Mg and Ag are displayed separately in the top view of the reconstructed volume in Figure 5c. Clearly, inspection of this figure reveals the enrichment of the β'/ matrix interface with Li, Cu and Mg atoms, as indicated by the arrows. However, the enrichment of Li and Cu is more pronounced than that of other solute atoms. To quantify the enrichment of the β'/ matrix interface by solute atoms, the composition profile is drawn in the cylinder perpendicular to the interface. According to this profile, the compositions of Li and Cu atoms at the interface show an increase of approximately 2 at. % and 5 at.%, respectively. However, it seems that Mg atoms concomitantly segregated with Li and Cu atoms, whereas Ag did not. This observation, in combination with the TEM image (Figure 2a), indicates the strong segregation behavior of the Li and Cu atoms at the interface. This segregation suggests that β' particles possibly favor preferred nucleation sites for the δ' phase or/ and T1 phase. As the Li content in the investigated alloy is less than 5 at.%, the presence of the δ' phase is not expected. On this basis, it can be concluded that β' dispersoids play a potential role as heterogeneous nucleation sites for the T1 phase in the naturally aged sample.
The good hardness response for this particular temper can be attributed to the presence of β' dispersoids, which is considered to be one of the hardening phases in a quenched sample [27]. Moreover, natural aging promotes a diffusion and segregation of solute atoms at the β'/ matrix interface. This segregation behavior can be quantified using the APT method, as described above.
3.7 Cold working and artificially aging of the samples at T8 temper
To provide a qualitative assessment of the effect of the heat treatment condition on the precipitation kinetics and phase transformation through the transition from naturally aging condition to peak state aging, an APT analysis for the sample following T8 heat treatment was performed (Figure 6). Areas of higher Cu concentrations are visible in Figure 6a. Isoconcentration surfaces, which delineate regions containing more than 4 at.% Cu, are included to aid in the visualization of the θ' precipitates with platelet morphology, an average diameter of (1.8 ± 0.2) nm and an average length of (11 ± 1) nm. Figure 6b shows a combined proxigram concentration profile based on the isoconcentration surfaces of all of the highlighted θ' precipitates.
Starting at the matrix/precipitate heterophase interface and moving into the precipitate, the chemical composition was determined to be (29.6 ± 1) at.% Cu, (3.2 ± 0.4) at.% Li and (1 ± 0.2) at.% Mg. Concomitantly, the Li concentration inside the precipitates was found to be the same as its value in the matrix. This observation indicates the partitioning of the Li atoms within the θ' precipitates, as shown in Figure 6a. The partitioning of Li atoms to the θ' precipitates might indicate the possible occurrence of the phase transformation of the θ' precipitates at the later stages of the aging. Given its importance for strengthening Al, a large number of studies of the Al-Cu precipitation sequence have focused on the properties of metastable θ' [28,29]. Relatively little is known concerning the compositional evolution of θ' precipitates because its nanometer-scale platelet-like morphology makes quantitative analytical electron microscopy analyses extremely difficult. A recent study by Biswas et al. [30] shows that θ' precipitates are observed upon aging between 190°C and 260°C in binary Al –1.7 at.% Cu alloys. They observed that after aging the alloy for 8 h at 190°C, θ' precipitates exhibit a range of Cu-deficient core concentration that differ from the equilibrium composition of (Al2Cu). In contrast, alloys aged at 260°C for 4 h exhibited precipitates with Cu core concentrations close to the equilibrium value. According to the authors, the cause of the Cu deficiency in the θ' precipitates at the lower aging temperature remains an open issue. In our study, the applied T8 heat treatment implies an artificially aging of the alloy for 30 h at 150°C. This aging temperature is below the metastable solvus boundary for the θ' precipitates in Al-Cu phase diagram [31], which justifies the presence of the θ' phase in the microstructure. The agreement of our compositional analysis of the θ' platelet with the expected stoichiometric composition of (Al2Cu) can be explained by considering the enhanced Cu diffusion to the existing defects in the microstructure with aging. Increase aging time results in a core concentration close to the equilibrium one.
In addition to the θ' precipitates, a variety of the T1 platelet precipitates are found within the microstructure (Figure 7). This observation is consistent with the reported result by Decreus et al. [32], who proposed that the pre-deformation process mostly promotes the formation of the T1 phase, which is associated with the precipitates from the Al-Cu binary system, i.e., the θ' phase. Note the fine distribution of the T1 platelets with an average thickness of (1.4 ± 0.2) nm and an average length of (11.8 ± 2) nm within the microstructure (Figure 7a). Moreover, the intersection of more thantwo platelets, as indicted by the lines in Figure 7a, can be observed. These reported observations in this temper condition can be attributed to the applied plastic deformation prior to the artificial aging. The plastic deformation results in a high dislocation density, which results in a high density of nucleation sites for the T1 and θ' platelets and the presence of a high initial vacancy flux, which leads to the enhanced diffusion of the solute atoms.
The observed microstructure and good mechanical properties at this temper confirm that applying plastic deformation accelerates the precipitation kinetics by approximately one order of magnitude [33]. Quantitative evaluations of the T1 platelets precipitates presentedin Figure 7b show that the average chemical composition, as estimated from the concentration composition profile based on the isoconcentration surface that contains more than 6 at.% Li (calculated over 20 precipitates containing in different reconstructed volumes), is (13±1) at.% Li, (11±1) at.% Cu, (3±1) at.% Mg and (1.5±0.4) at.% Ag. This value shows a deviation from the (Al2LiCu) stoichiometry of the bulk T1 phase and the enrichment of this phase with Mg and Ag. The reason behind the deviation from the (Al2LiCu) stoichiometry has been reported in different studies [34,35]. The enrichment of the T1 platelets with Mg and Ag play an important role in the high harness value for the specimen in this condition. By considering the current model proposed by Cassada et al. [36], the precipitation of the T1 phase on the {111} Al planes requires the presence of one or more partial dislocations bounded by a stacking fault. Because the stacking fault energy (SFE) of Al is very high, the dissociation of dislocations into partials, which is believed to be necessary for nucleating the T1 phase, can occur. This process can be facilitated by elements known to decrease the SFE of Al; among these elements, Mg and Ag have been found to be highly effective [37]. Regarding the effect of Mg and Ag enrichment inside the T1 platelet on the mechanical properties of the alloy, the single addition of Ag in Al- Cu-Li-Zr alloys was found to have no effect on the age hardenable curve, while Mg addition significantly raises the peak hardness and shortens the time to the peak [19]. The peak hardness further increases when Ag is added together with Mg. This effect is explained as follows. In the case of single addition of Ag, the T1 platelet only nucleates on the dislocation loop around a Zr dispersoid. In contrast, Mg addition causes the formation of octahedral micro-voids and new GP zones on {111} Al planes; these structures act as nucleation sites for the T1 phase, providing a fine dispersion and hence an increase in the hardness of the system. Moreover, the intersection of the T1 platelets confirms that theses platelets favor a mechanism to hinder the movement of dislocations resulting in high hardness value (Figure 1). The suppression of dislocations glides by second-phase particles, i.e., T1 precipitates in this case, will increase the stress required for the dislocation to move through the particles, which should also increase the yield strength [27].
To follow the precipitation kinetics and phase transformations at the later stage of the aging process, the samples in the T8 heat treatment condition were further aged for 12 h at 150°C. The reconstructed volume of this sample after LAWATAP analysis is shown in Figure 8. In this Figure, only one T1 platelet is observed, with a thickness of 2 nm and length of 62 nm in the [111] direction. This phase is also observed to be enriched with Mg and Ag, which indicates thatboth Mg and Ag are associated with coarsening resistance of the T1 phase. The small thickness and large length of the observed T1 platelet can be explained in terms of the high coherency of the edge of the T1 precipitates with the {111} Al atomic planes. The final layer of the structure proposed for the T1 platelet precipitates is found to have an extremely low lattice mismatch with the {111} Al atomic planes [38]. The observation in Figure 8 confirms that the T1 platelet grows via a ledge mechanism [36], whereas both of the board faces of the T1 platelet are coherent with the matrix. The value of the measured thickness of this platelet indicates that no thickening of the T1 platelet is observed. It is surprising to observe the absence of the θ' platelets in the microstructure. This observation suggests the occurrence of the phase transformation in the form of the dissolution of the θ' phase upon further aging.
3.8 Artificially aging of the samples at T6 temper
Microstructure evaluation at the atomic scale was performed for the sample under the T6 heat treatment condition. In an effort to accelerate precipitation kinetics without applying plastic deformation, artificial aging is performed at a higher temperature, 180°C in this case. After a long aging time of 20 h, the microstructure is found to be predominantly T1 platelets (Figure 2c). The reconstructed volume of the sample in T6 condition analyzed by LAWATAP is shown in Figure 9. In this case, the microstructure has a uniform distribution of the T1 platelets, with an average thickness of (4.7± 1) nm and an average length of (40± 2) nm, without any indication of their intersection (Figure 9a). A combined proxigram profile corresponding to theses T1 platelets, which are delineate by 5 at.% Li isoconcentration surfaces, is shown in (Figure 9b). As illustrated in this figure, the chemical composition of this phase in this temper condition was found to be (15.7±1) at.% Cu, (16.6±2) at.% Li, (2.4±1) at.% Mg and (1.4 ±0.4) at.% Ag. It is worth notable that the enrichment of the T1 phase with Mg and Ag is similar to that of the specimen in the T8 condition. The presence of the T1 platelets and their enrichment with Mg and Ag are responsible to the age-hardenable behavior under his condition (Figure 1).
Moreover, an inhomogeneous distribution of Cu and Mg was observed in the Al matrix Figure 9c. The locations of Mg-enriched regions coincide with the Cu- and Li- enriched regions. Quantitative evolution of these enriched regions was performed through the proxigram composition profile from the isoconcentration surface of 0.5 at.% Mg (Figure 9d). According to this proxigram, the chemical composition at these enriched regions was estimated as (7.1±3) at.% Cu, (3.8±1) at.% Li, (4.7±1) at.% Mg and (0.5 ±0.2) at.% Ag. From this dataset, the growth of the T1 platelets in the Mg enriched region, which contains localized Ag atoms, is clearly observed. This observation suggests the potential role of Mg and Ag to catalyze the nucleation of the T1 phase. Mg and Ag are typically used to increase the number of dissociated dislocations and thus the nucleation sites of the T1 phase [39]. Moreover, it has been reported that Mg atoms enhance the equilibrium vacancy concentration during solution treatment and help to retain these vacancies during the quenching operation. On this basis, the vacancies will provide a dual beneficial effect; enhanced nucleation and increase diffusivity [40]. This effect can be seen clearly in Figure 9c. However, the distribution of these elements in the structure of the T1 platelets as well as their portioning between precipitates and matrix is the subject of conflicting reports [41].
4. Conclusion
The influence of different industrial heat treatments, namely, the T4, T8 and T6 tempers, on the precipitation kinetics and phase transformation of an Al-Li alloy was investigated. Alloy AA2195 was selected as a model system in the present study. A detailed analysis of the correlation between the microstructure developments due to the respective treatment conditionand the hardening process revealed the following:
- The microstructure of the sample undergoing the T4 heat treatment reveals the enrichment of the β'/ matrix interface with Li, Cu and Mg atoms. This observation indicates thepotential role of the β' phase as the nucleation site for the T1 phase. The segregation of solute atoms to the β'/ matrix interface was quantified.
- After conducting the T8 heat treatment, the fine distribution of the θ' and T1 phases within the microstructure was observed. The intersections of different platelets were used to suppress the movement of the dislocations, resulting in a high hardness value for the specimen. The growth of the T1 platelet precipitate via a ledge mechanism was observed by increasing the aging temperature. The absence of θ' platelets from the microstructure after increasing the aging temperature suggests the occurrence of phase transformation in the form of the dissolution of the θ' phase with increasing aging temperature.
- With the T6 heat treatment, the microstructure was dominated by the T1 phase, which nucleated on Mg enriched regions that contain localized Ag atoms. The Mg atoms play an important role to enhance the nucleation of the T1 phase at this heat treatment condition.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
Author Contributions
Muna Khushaim Conceived the study, performed the experiments,
analyzed the data and wrote the paper.
Prof. Alexander Rothenberger supervises the experimental work,
reviewed and edited the manuscript.
Acknowledgments
The Authors thank Judith Seibert (Institute for Physics, University Augsburg,) for her support with transmission electron microscopy. Prof. Talaat Al-Kassab is gratefully acknowledged. M. Khushaim gratefully acknowledges financial support provided through King Abdullah University of Science and Technology (KAUST) base-line funding program.