1. Introduction
Glutathione (GSH) is a tripeptide (γ-L-glutamyl-L-cysteinylglycine) characterized by an atypical peptide bond linking the nitrogen of cysteine to the carboxyl in γ-glutamic acid. GSH is found ubiquitously in nature, both in animals and in plants and microorganisms. Its ubiquitous distribution virtually in all kingdoms of life suggests that GSH plays a fundamental role in the cell and may indicate a conserved role in evolution.
GSH is present in the cell at millimolar concentrations and plays a focal role in the thiol-based cell redox signaling, protein function, gene expression, cell differentiation, and cell proliferation. It is the main thiol compound with the lowest molecular weight present in both animal and plant cells. GSH plays a major role in the detoxification of various compounds, including xenobiotic such as drugs, endogenous metabolic products, and toxic metals. Of utmost importance, it acts as an intracellular antioxidant, protecting against oxidative stress, particularly that generated by lipid peroxidation [1-2]. One of its functions is to maintain in the reduced state the -SH groups of many enzymes and proteins whose oxidation leads, in most cases, to inactivation or loss of the biological function.
GSH deficiency may be associated with oxidative stress, a condition that may play a key role in aging and in the pathogenesis of many diseases, such as cancer, diabetes, neurodegenerative and cardiovascular diseases [3]. GSH may exist in the cell in two forms: reduced glutathione (GSH) and oxidized glutathione (GSSG), which are in a constant dynamic equilibrium. Chronic oxidative stress reduces the cell levels of GSH, and it is often appropriate to replenish its levels. Increasing GSH plasma levels may have beneficial systemic effects and may be of therapeutic relevance. GSH intake via the oral route does not successfully enhance GSH in plasma, due to its metabolization in the gut where it is hydrolyzed into its three constituent amino acids by a γ-glutamyl transpeptidase (γ-GT) present in the intestine. It is therefore necessary to use a high oral dose in order to guarantee significant absorption. Witschi et al. evaluated the increase in the blood levels of GSH, cysteine and glutamate after oral administration of GSH to seven healthy volunteers. No significant increases were observed at doses of up to 3 g per dose [4]. Sublingual administration can be used as an alternative to oral administration of GSH being glutathione absorbed by buccal mucosal cells in vivo [5]. Prophylaxis based on GSH is used in some cases by parenteral, intramuscular or slow intravenous administration for neuropathy resulting from chemotherapy with cisplatin or analogues [6]. Many attempts have been made to develop GSH derivatives able to easily cross the cell membranes and to enhance its oral bioavailability. Current strategies attempting to improve GSH bioavailability are based on the prodrug approach, using GSH prodrugs or derivatives, like N-acetylcysteine (NAC), a well absorbed precursor of cysteine, the main limiting factor in GSH synthesis [2,7-8]. Currently, NAC administration has been the only therapeutic strategy against oxidative stress-associated disorder in HIV infection, even if the efficacy of NAC to increase intracellular GSH levels is correlated to the ability to synthesize GSH intracellularly by its precursors, a process often impaired by aging process, viral infections and liver diseases [8-10]. A promising approach is represented by GSH esters prodrugs, that just after enter the cell are hydrolyzed to GSH thus by-passing the de novo synthesis of GSH [8,11].
S-Acetylglutathione (SAG) is a GSH precursor, it is more stable than GSH itself in plasma and is taken up directly by cells and later converted to GSH (see Figure 1). The acetylation of the sulfur atom prevents the decomposition of GSH and facilitates its absorption through the intestinal wall as is, thus enabling the molecule to pass extensively into the cells. Moreover, cysteinyl acetylation prevents the oxidation of the thiol group before its absorption. After absorption, SAG is hydrolyzed by cytoplasmic thioesterases [8-9], so releasing a GSH pool available for the cells. The addition of SAG to cultures of fibroblasts originating from individuals suffering from a genetic glutathione synthetase deficiency has proved able to replenish the intracellular level of GSH effectively [12]. SAG has proven to be more stable in plasma and more effective than GSH in replenishing the cell levels of GSH depleted by viral infections [7,9]. Moreover, SAG exhibits an interesting non-GSH-dependent activity that induces apoptosis in some human tumor cell lines in vitro [13].
Emothion® is an S-acetylglutathione produced by Gnosis by a chemical acetylation of the thiol group on the cysteinyl amino acid. In this study we compared the bioavailability of Emothion® and a reference marketed GSH product in a single center, single dose, randomized, open-label, two-sequence, two-period, cross-over clinical trial on eighteen healthy volunteers.
2. Materials and Methods
2.1 Chemicals
UPLC-grade acetonitrile, UPLC-grade acetonitrile, methanol and water were purchased from VWR (Rome, Italy). Dichloromethane was from Sigma Aldrich (Milan, Italy). All other chemicals were analytical grade reagents from Sigma Aldrich. GSH and SAG standards (purity 99%) were provided by Gnosis SpA.
2.2 Test and reference products
3.494 g of Emothion®, S-acetyl glutathione (SAG) sachets (test product), were produced by Gnosis S.p.A. (Desio, Italy). SAG was furnished as a pure powder. The content of one sachet was poured and dissolved in 300 mL of still mineral water immediately before administration. Setria® L-Glutathione (reference product), 500 mg capsules, was purchased from Kyowa Hakko USA (New York, USA). Seven capsules were administered consecutively with 300 mL of still mineral water. One single dose of either test or reference product was administered in one of the two cross-over periods according to the randomization list.
2.3 Clinical study design
This was a single centre, single dose, randomized, open-label, two-sequence, two-period, cross-over bioavailability study. The study protocol, the Investigator’s brochure and all other relevant documentation were reviewed and approved by an independent Ethics Committee (Comitato Etico Cantonale, Canton Ticino, Switzerland). The study was performed in accordance with the Declaration of Helsinki and to the general principles of ICH Harmonised Tripartite Guidelines for Good Clinical Practice.
Primary endpoint was to describe the pharmacokinetic profile of GSH in plasma after single dose administration of test and reference product. Secondary endpoints were: to describe the pharmacokinetic profile of SAG in plasma after single dose administration of the test product, to assess the concentration levels of the GSH-related dipeptides Cys-Gly and γGlu-Cys in plasma after single dose administration of test and reference products and to collect safety and tolerability data of test and reference products after single dose administration. In addition, the pharmacokinetic profile of GSH after single dose administration of test and reference product was also measured in erythrocytes.
Eighteen (18) male and female subjects, aged 18-55, were included in the study after signing written informed consent. Inclusion criteria were as follows: body mass index between 18.5 and 25 kg/ m2; normal vital signs (systolic blood pressure 100-139 mmHg, diastolic blood pressure 50-89 mmHg, heart rate 50-90 bpm); normal clinical laboratory values. Exclusion criteria included: abnormally high homocysteine levels; known methyl-tetrahydrofolate reductase (MTHFR) and cystathionine β-synthase (CBS) gene polymorphism; ascertained or presumptive hypersensitivity to the active principles; history of anaphylaxis to drugs or allergic reactions in general, which the investigator considered could affect the outcome of the study; significant history of renal, hepatic, gastrointestinal, cardiovascular, respiratory, skin, haematological, endocrine or neurological diseases that could interfere with the aim of the study; medications, including over the counter medications, nutraceuticals or food supplements containing GSH or SAG or amino acids, particularly sulfurated amino acids (Cys), multivitamin dietary supplements containing any other antioxidant compounds, and herbal remedies for 2 weeks before the start of the study; participation in the evaluation of any investigational product for 3 months before this study; blood donations for 3 months before this study; history of drug, alcohol [>1 drink/day for females and >2 drinks/day for males, defined according to the USDA Dietary Guidelines 2015], caffeine (>5 cups coffee/tea/day) or tobacco abuse (≥10 cigarettes/day); abnormal diets (<1600 or >3500 kcal/day) or substantial changes in eating habits in the 4 weeks before this study; intense physical activity in the 3 days before the screening visit; positive or missing pregnancy test at screening or day -1, pregnant or lactating women. Females of child-bearing potential had to be using at least one reliable method of contraception.
Patients were randomly allocated to one of two sequences of products in the two study periods according to the randomisation list and cross-over design. Seventeen (17) out of 18 treated subjects completed the study as per protocol. The number of 18 subjects was deemed sufficient for the descriptive purpose of the study, but the sample size was not based on any statistical calculation.
After an overnight fasting condition, an indwelling catheter was placed in an antecubital vein and three blood samples were collected every 5 min to establish baseline levels of GSH. The 18 subjects enrolled in the trial were divided into two arms (9 subjects each) each one receiving a single oral dose of 3.494 g of test or 3.5 g of reference products under fasting conditions in two subsequent periods, separated by a wash-out interval of at least 7 days, exchanging the treatment in second period according to a randomized cross-over design. Blood was drawn at 15 min, 30 min, 1h, 2h, 3h, 4h, 8h, 12h and 24h.
2.4 Sample treatment
After collection, samples were centrifuged and plasma was aliquoted and immediately frozen at -80°C. The buffy coat layer of white blood cells was removed with the tip of a pipette and erythrocytes pellet was immediately divided into 2 aliquots and frozen at -80°C. Samples were analyzed within 3 months from collection.
An aliquot of plasma (500 μL) was treated with tributyl-phosphine (50 μL of a 10% solution in DMF), then acidified with two volumes of 10% metaphosphoric acid. After 30 min at 4°C, samples were centrifuged at 15000g and 4 °C for additional 30 min. 500 μL of the supernatant were extracted with 1 mL of dichloromethane by extensive vortexing. After centrifugation at 15000g for 30 min, 300 μL of supernatant were lyophilized and resuspended in 200 μL of formic acid 0.1%. 10 μL were analyzed by UPLC/MS.
2.5 Analytical methods
For the determination and quantification of GSH, SAG, GSSG, γGlu-Cys and Cys-Gly in the extracted plasma samples, a Waters ACQUITY UPLC system (Waters, Milford, MA) was utilized, including a quaternary solvent manager (QSM), a sample manager with flow through needle system (FTN), a photodiode array detector (PDA) and a single-quadruple mass detector with electrospray ionization source (ACQUITY QDa). Chromatographic analyses were performed on a BEH C18 column (2.1 x 50mm i.d., 1.7 μm particle size) thermostated at 25°C. Solvent A was 0.1% aqueous HCOOH and solvent B was 0.1% HCOOH in CH3CN. Flow rate was 0.5 mL/ min and elution was performed as follows: 10% A for 1 min, then B was linearly increased up to 100% in 4 min and 100% B for 2 min. The column was re-equilibrated with 90% B and 10% A before next injection. Mass spectrometric detection was performed in the positive electrospray ionization mode using nitrogen as a nebulizer gas. Analyses were performed in Single Ion Recognition (SIR) mode at the following m/z values: 308 m/z for GSH (Rtime = 0,55 min); 251 m/z for γGlu-Cys (Rtime = 0,5 min); 350 m/z for SAG (Rtime = 2,1 min); 179 m/z for Cys-Gly (Rtime = 0,3 min). Capillary voltage was 0.8 kV, cone voltage 15 V, ion source temperature 120 °C and probe temperature 600 °C. Identification of the peptides of interest was made by using analytical standard compounds. The instrument control and data acquisition were carried out using the Waters Empower 3 software.
For the determination of GSH in erythrocytes, an HPLC with fluorometric detection method was employed. Erythrocyte pellet was lysed in 4 volumes of water and treated with tributyl-phosphine (50 μL of a 10% solution in DMF). Cells were lysed by freezing and thawing for three times and then proteins were precipitated with two volumes of 10% metaphosphoric acid. After 30 min at 4 °C, samples were centrifuged at 15000g and 4 °C for additional 30 min. An aliquot of 20 μL of supernatant was diluted with 300 μL of 0.1 M phosphate buffer, pH 8.0, containing 0.1% EDTA and then derivatized with 20 μL of o-phthaldialdehyde (OPA) solution (1 mg/mL in methanol). After incubation at room temperature for 15 min in the dark, 50 μL of the reaction mixture was analysed by HPLC with a Waters 60F pumps and 600 pumps control unit system equipped with a X-Bridge C18, 5 μm, 4.6 × 150 mm column associated with a Symmetry C18, 3.9 × 20 mm guard column (Waters Corporation, Milford, Massachusetts, USA), and a Waters 71P auto-sampler. The mobile phase consisted of 15% (v/v) methanol in 25 mM Na2HPO4, pH 6.0. Isocratic elution was performed at 37°C at a flow rate of 0.6 mL/min. The excitation/ emission wavelengths were set to 350/420 nm in a Shimadzu RF- 551 spectrofluorometric detector. The instrument control and data acquisition were carried out using the Waters Millennium32 software. GSH intracellular levels, normalized for haemoglobin content, were expressed as micromoles of GSH per millimole of haemoglobin.
2.6 Statistical methods
The trial and the methodologies for statistical comparison between test and reference formulations were designed taking into account the recommendations of the guidance on the investigation of bioequivalence (CPMP/QWP/EWP/1401/98 Rev. 1, 20 January 2010) [14] although the demonstration of a bioequivalence was not among the objectives of the present trial.
The pharmacokinetic analysis and the statistical analysis of pharmacokinetic parameters were performed using Phoenix WinNonlin® version 6.3, Pharsight Corporation, and SAS® version 9.3 (TS1M1). The statistical analysis of safety data was performed using SAS® version 9.3 (TS1M1). The data documented in this trial and the clinical parameters measured were analysed using classic descriptive statistics for quantitative variables and frequencies for qualitative variables. For GSH, baseline-corrected pharmacokinetic parameters AUC0-t and Cmax, if feasible, were compared between test and reference using analysis of variance (ANOVA) for a cross-over design on log-transformed data. Period, treatment, sequence and subject within sequence were taken into account as sources of variation. tmax was compared between treatments, if feasible, using the non-parametric Wilcoxon signed-rank test.
3. Results
3.1 Plasma GSH, Cys-Gly and γGlu-Cys levels
The concentration of GSH, SAG and of the two dipeptides Cys-Gly and γGlu-Cys was determined in plasma at the following times: -1h, -30 min and -5 min (pre-dose), 15 and 30 min, 1h, 1.5h, 2h, 3h, 4h, 8h, 12h and 24 h post-dose. Multiple predose analyses were performed to evaluate individual variations in the baseline level of GSH.
Primary variables examined were baseline-corrected Cmax and AUC0-t of GSH in plasma after single dose administration of test and reference products, whereas the secondary variables were: i) baselinecorrected AUC0-∞, tmax, t1/2 and Frel of plasma GSH after single dose administration of test and reference products; ii) Cmax, AUC0-t, AUC0-∞, tmax and t1/2 of SAG in plasma after single dose administration of the test product; iii) Cys-Gly e γGlu-Cys levels after single dose administration of test and reference products.
Mean (±SD) plasma glutathione concentrations (μM) vs. time profiles after single dose of test and reference compounds are shown in Figure 2. SAG was not quantifiable in any plasma sample. This result shows that deacetylation of SAG occurs rapidly after the administration and that the whole amount of the substance is transformed into GSH before reaching the blood stream.
Main pharmacokinetic parameters of plasma GSH are summarised in the following Table 1.
mean±SD is reported except for tmax for which median (range) is shown.
On average, GSH plasma concentrations showed higher Cmax and AUC0-t after single dose of the test than the reference formulation. Median tmax was 1.5 h post-dose after test and reference formulation.
The mean relative bioavailability of plasma GSH calculated as AUC0-t T/R ratio (Frel) was 1325.04 ± 2936.84%. Frel had a remarkably high variability (CV%=221.64%). Indeed, the parameter ranged from the minimum 7.42% to the maximum 11378.96% which was more than 1550 times higher than the minimum.
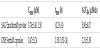
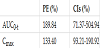
The outcome of the statistical comparisons shows a significant difference between the test and the reference formulations (Table 2). GSH plasma concentrations showed significantly higher rate (Cmax) and extent (AUC0-t) after single dose of SAG powder than the reference formulation, whereas Tmax of the test and the reference products was not significantly different (p value=0.5276, Wilcoxon test). Provided that Point Estimate (PE) for bioequivalent compounds is 100%, the reported values in this case are an evidence of a significant difference. Confidence Intervals (CI) indicate an equivalence for values ranging between 80% and 120%. In this case, the range found is significantly different, especially for AUC parameter.
Differently from GSH, no significant variation in the plasma concentration of either Cys-Gly or γGlu-Cys was appreciable after either the test or the reference.
3.2 Erythrocytes GSH levels
The concentration of GSH was determined in erythrocytes at the following times: -1h, -30 min and -5 min (pre-dose), 15 and 30 min, 1h, 1.5h, 2h, 3h, 4h, 8h, 12h and 24 h post-dose. Multiple predose analyses were performed to evaluate individual variations in the baseline level of GSH.
Pharmacokinetics variables examined were baseline-corrected Cmax, AUC0-t and Frel of GSH after single dose administration of test and reference products.
Mean (+SD) GSH concentrations in erythrocytes (GSH/Hb) vs. time profiles after single dose of test and reference compounds are shown in Figure 3.
Main pharmacokinetic parameters of glutathione in erythrocytes are summarised in the following Table 3.
On average, Cmax and AUC0-t after single dose of the reference formulation were slightly higher than after the test. The mean relative bioavailability of GSH in erythrocytes calculated as AUC0-t T/R ratio (Frel) was 224.17±480.48%. Frel had a remarkably high variability (CV%=214.34%). Indeed, the parameter ranged from the minimum 0% to the maximum 1924.99%.
The outcome of the statistical comparisons of the PK parameters between T and R is summarized in Table 4.
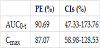
The data show a significant difference between the test and the reference formulations both in terms of Cmax and AUC0-t of GSH in erythrocytes. After single dose of the reference capsule formulation, GSH had slightly but significantly higher rate (Cmax) and extent (AUC0-t) of absorption in erythrocytes whereas Tmax of the test and the reference products was not significantly different (p value=0.6970, Wilcoxon test).
3.3 Safety of the formulations
No adverse events were registered as assessed by vital signs (blood pressure and heart rate), physical examination, body weight and laboratory tests.
The safety and tolerability profile of SAG powder was excellent. In particular, three unrelated treatment emergent adverse events (TEAEs) were reported. No significant change in subjects’ baseline conditions (vital signs, BW, clinical laboratory assays) was observed after single dose of either formulation.
Overall, three TEAEs occurred during the study (frequency of 11.1% corresponding to 2 out of 18 subjects). One TEAE was reported after administration of the test formulation and two TEAEs after administration of the reference formulation. The reported TEAE were unrelated to the treatment. No serious adverse events (SAEs) occurred during the study. One subject discontinued the study due to two TEAEs, i.e. due to episodes of abdominal pain of moderate severity and pyrexia during the wash-out period after receiving the reference formulation. No clinically meaningful effect on vital signs, BW or laboratory parameters was observed.
4. Discussion
There is growing interest in research aimed to find new potential therapeutic or nutritional approaches to maintain or increase GSH levels, in order to improve antioxidant defenses to counteract both acute and chronic diseases and oxidative damage occurring with aging. Because of its biochemical and pharmacokinetic properties, the use of oral GSH as therapeutic agent is currently doubtful. In fact, GSH has a very short half-life in human plasma (about 1.6 min), it undergoes to hydrolysis and oxidation during the digestion process also interacting with plasma proteins. Moreover, GSH cannot cross cell membranes, but it has to be broken down by the intestinal enzyme γGT into single amino-acids and then resynthesized from them intracellularly, mainly in the liver [2,9,10,15]. For these reasons, in the pharmaceutical field, prophylaxis based on GSH is generally obtained by parenteral, intramuscular or slow intravenous administration, and, in case of oral administration, it is possible to reach a desirable therapeutic effect only with high doses, trying to overcome its poor absorption.
Despite oral GSH administration could be the easiest way to restore or maintain GSH levels, it is not often used in therapy because of its questionable efficacy. Indeed, while many studies on oral GSH supplementation showed promising results in animal models, with increase of plasma and tissue GSH levels [16-17] which enhanced immune function and protection against aging-related impairments, influenza infections and cancer [18-20], the efficacy in humans is less convincing. Indeed, oral GSH supplementation in healthy adults reported no significant changes in GSH status and in biomarkers of oxidative stress [4,21]. Conversely, studies evaluating the effect of GSH supplementation associated with chemotherapeutic agents in patients with cancer underlined a valuable effect from oral GSH, showing a reduction of adverse effects linked to the oxidative action of the chemotherapy without affecting the efficacy of the treatment [6,22-23]. More recently, a randomized, double-blinded, long-term placebo controlled study demonstrated that GSH oral supplementation in humans increased GSH levels in various body stores [20]. In another randomized crossover trial, involving 20 volunteers with metabolic syndrome, Schmitt et al. demonstrated the efficacy of a new sublingual form of GSH, both in terms of bioavailability and capability in counteracting oxidative stress [10]. This different absorption of oral GSH between humans and mice could be explained by the different quantity and activity of the intestinal γGT enzyme [4].
Even if GSH esters are able to increase mitochondrial GSH, some studies revealed significant toxicity in vitro [24-25]. More recently, another promising strategy is represented by using S-acyl prodrugs, like SAG, able to cross the cellular membrane and then easily converted intracellularly to GSH by cytoplasmatic thioesterases [8-9]. SAG is more stable in plasma than GSH [26] and it is reported to be able to increase intracellular GSH in rat brain synaptosomes [27] and in cultured fibroblast cells from patients with Glutathione synthetase deficiency (GSD) [12]. Moreover, SAG was shown to restore GSH levels decreased due to HSV-1 infection [9,28]. Furthermore, Fraternale et al. [7] showed that SAG is able to reduce murine AIDS typical signs with potent antioxidant and antiviral properties, making it possibly efficacious in combination with antiretroviral drugs in the treatment of retroviral infections.
Taking together all these considerations, we decided to study the bioavailability, measured as PK profile, of a new oral SAG entity (Emothion®, Gnosis) in comparison with a marketed GSH product (as reference product) after single dose administration in healthy volunteers, determining their respective ability in increasing GSH levels in plasma and erythrocytes.
We conducted this clinical trial on eighteen subjects, all receiving at least one dose of investigational products, with seventeen of them that completed the study. The dose of 50 mg/kg, corresponding to 3.5 g in case of a representative person weighing 70 kg, was chosen, according to a phase I PK study published by Park et al. [29]. This is a higher dose than the daily suggested one of 500 mg/day, even if doses of 1000 mg/day of GSH are also used. However, it has been already administered as single dose to human subjects without any drawbacks for the volunteers. The use of high doses is necessary to evaluate the absorption profile of GSH and SAG, given that the endogenous background GSH could mask the increase GSH following oral supplementation. The high dose is justified by the previous study by Park et al. [29] and was chosen in order to highlight an effect.
This kind of study was quite difficult to carry out, being GSH also produced endogenously and strictly regulated; indeed, various studies conducted by other groups did not evidenced any increase of GSH levels or modulation of oxidative stress markers after single dose oral supplementation of GSH to healthy subjects in either plasma and erythrocytes [4,21,29].
In the present trial, SAG showed very high tolerability and safety, with only three TEAEs reported, corresponding to the 11.1% of 18 subjects, all of these unrelated to the treatment. No SAEs occurred during the study and only one subject discontinued the study due to episodes of abdominal pain of moderate severity and pyrexia during the wash-out period after receiving the reference (GSH) formulation. Furthermore, no significant change in subjects’ vital signs (blood pressure and heart rate), physical examination, body weight and clinical laboratory assays baseline conditions was observed after either formulation administration.
In order to achieve a complete evaluation of the effect of SAG supplementation on body GSH stores, we measured GSH levels in plasma and erythrocytes. Indeed, blood GSH is concentrated in erythrocytes, but lower levels can also be found in plasma; moreover, we evaluated also GSH-related dipeptides Cys-Gly and γGlu-Cys in plasma.
We optimized a UPLC-MS method for the determination and the quantification GSH, SAG, GSSG, γGlu-Cys and Cys-Gly in plasma samples, given their very low levels in this matrix. For the determination of GSH in erythrocytes, an HPLC with fluorometric detection was employed, because of the high concentration of GSH in erythrocytes.
We found that plasma SAG levels were not quantifiable in any samples, due to the rapid deacetylation of the whole amount of SAG to GSH after the administration before reaching the blood stream. Only total (free, oxidized and protein bounded) GSH levels were measured in plasma since the in vivo levels of plasma GSH are low and the majority of GSH is present in the oxidized disulfide state or linked to proteins [20,30]. Thus, plasma samples were first reduced with tributylphosphine prior to protein precipitation with metaphosphoric acid, to allow the measurement of total GSH.
Analyzing all the PK variables listed in methods sections, we noted that GSH concentrations showed a small increase and a low peak followed by a decline up to 24 h post-dose, both in plasma and in erythrocytes, in a similar way after single dose of either SAG or GSH used as reference formulation. Interestingly, GSH showed significantly higher rate (Cmax) and extent (AUC0-t) of absorption in plasma after single dose of SAG than after the reference GSH product, whereas, in erythrocytes, these parameters were slightly though significantly higher after a single dose of the GSH than after SAG. GSH concentration in erythrocytes varied slightly from the time of administration up to 24 h post-dose with low variations between SAG and GSH reference product. Tmax of both formulations did not significantly differ between treatments either in plasma or erythrocytes. Erythrocytes are a tissue where GSH is accumulated in the body. Erythrocytes GSH levels are higher than plasma level and are probably not or poorly affected by a short time modification in GSH concentration like that occurring after a single dose administration. Moreover, the metabolism of GSH and GSSG in erythrocytes is very complex and regulated by many factors.
The reason why, in our study, erythrocytes GSH level is slightly lower in subjects administered with SAG remains to be better elucidated.
To achieve a more complete comprehension of GSH metabolism after single dose administration, we also analyzed the plasma levels of the GSH-related dipeptides Cys-Gly and γGlu-Cys after single dose administration of test and reference products, finding no evident variation appreciable after single dose of either formulation, confirming data in literature. Furthermore, the found basal levels of Cys-Gly and GSH in this study are in agreement with literature data, confirming a wide variability among individuals [30-32].
Actually, restoring GSH levels is a challenging and desirable effect only achievable with the use of high doses of oral GSH because of its unfavorable biochemical and pharmacokinetic characteristics. In this study, we showed that a single oral dose administration of a GSH prodrug, SAG, is able to significantly increase the rate and the extent of GSH absorption. SAG is more stable than GSH, can be directly taken up by the cells and requires only cytoplasmic thioesterase for its hydrolysis to GSH with no need to be broken down to aminoacids that are, then, combined to synthetize GSH. This is a very important and useful in nutritional approach aiming to increase antioxidant defense and in all these cases where GSH catabolism and re-synthesis could be markedly affected, as in the case of viral infections or GSD, when the supplementation with NAC is less efficacious.
Competing Interests
The authors declare that they have no competing interests.