1. Introduction
Calcium phosphates, such as tricalcium phosphate (TCP) and hydroxyapatite (HAp), have been used as an implant material, because of its similarity with bone and teeth of vertebrate. On the other hand, HAp is widely used as a column packing material for affinity chromatography to separate various proteins and in industrial catalysts [1,2] because HAp absorbs proteins, amino acid and other substances [3]. Therefore, bacteria can be also favorable to adhere the surface of HAp and to form the biofilm.
Bacterial adhesion causes implant failure and implant removal, leading to patient suffering. To reduce the incidence of implantassociated infections, biomaterial surface treatments have been proposed [4]. Ceramics, plasma-deposited polymers, and hydrogels have been reported as a carrier coatings for bactericidal materials. Additionally, antibacterial compounds, such as antibiotics, antimicrobial peptides (AMPs), elements (silver, copper, and zinc), and organic cationic compounds have been widely used for surface modification [5]. Among them, AMPs are currently being considered as potential alternatives for antibiotics [6].
In this study, we focused on the immobilization of AMPs, especially, cationic antimicrobial peptide (CAP), protamine [7-9]. Protamine has 20 residues being arginine and is the most highly cationic naturally occurring CAP [7]. The bactericidal effect of many CAPs is thought to be due to the action on the cytoplasmic membrane of the susceptible bacteria, possibly through the formation of pores or destabilization of the membrane bilayer structure leading to lysis of the cell [8]. Previous studies showed that treatment of protamine on bacteria induced the cellular aggregation, pore formation, and complete lysis of the cell envelope [10]. In the present study, using electrostatic interaction between protamine and HAp, we have prepared the protamineadsorbed CPMs as a carrier for antibacterial material. We report the properties of protamine-loaded CPMs and antibacterial activity for gram- positive and gram-negative bacteria.
2. Materials and Methods
2.1 Preparation and characterization of calcium phosphate microspheres
As previously reported [11], the ultrasonic spray pyrolysis system consisted of an atomizer, a heating section (mullite tube: an ID of 2.5 cm and a height of 1 m; electric furnace: an ID of 3 cm and a height of 60 cm), a powder collecting section (test-tube filter), and a controller. The heating section consisted of two electric furnaces: a lower furnace for evaporating the solvent from the droplets (temperature: 300°C) and an upper furnace for pyrolysis (temperature: 850°C). An aspirator was used to introduce droplets into the heating section at a suction rate of 1.5 dm3•min−1. The starting solutions were prepared by mixing 0.60 mol•dm-3 Ca(NO3)2•4H2O, 0.40 mol•dm-3 (NH4)2HPO4 and 0.40 mol•dm-3 HNO3. Sample powders were prepared by spray pyrolyzing the starting solution using the ultrasonic vibrator with a frequency of 2.4 MHz. The as-prepared powders were washed with pure water to remove the excess nitrate (hereafter, washed powders). The crystalline phases of the resulting powders were identified using powder X-ray diffractometry (XRD; UltimaIV, Rigaku Co., Japan) with Cu-K radiation generated at 1645 kV and 200 mA. Data were collected in the range 2 = 10–50° with a step size of 0.02° and a counting time of 1 min/step. The crystalline phase was identified using the JCPDS reference patterns for β-TCP and HAp (#09-0169 and #9-0432). The morphology of the sample powders and cements was observed by scanning electron microscopy (SEM; JSM-6390LA, JEOL, Japan) at an accelerating voltage of 15 kV. Specimens for SEM observation were prepared by coating with Pt using sputtering prior to SEM observation. Ultrastructural analysis of the resulting particles was performed using high-resolution transmission electron microscopy (HR-TEM; JEM- 2100F, JEOL) at an accelerated voltage of 200 kV. HR-TEM samples were prepared by dispersing the powders in ethanol and collecting them onto carbon-coated copper grids (300 mesh, Agar Scientific, England).
2.2 Adsorption of protamine to microspheres and fabrication of discs
Protamine sulfate solutions (0-2000 mg•dm-3) were diluted by phosphate-buffered saline (PBS). Washed powders (1.5 g) were added into protamine solution (45 cm3) and incubated for 48 h at room temperature. The samples were centrifuged for 15 min at 8,000 rpm and the supernatant was assayed for protein using Bio-Rad protein assay following manufacturer’s instructions. Powders were washed with PBS 5 times and freeze-dried. The resulting powders (0 or 1000 mg•dm-3, 0.30 g) were uniaxially compressed at 50 MPa to form compacts (diameter: 15 mm; thickness: 1–2 mm) for antimicrobial evaluation.
2.3 Characterization of protamine loaded-microspheres
The zeta potentials of protamine loaded- CPMs powders were measured at 25°C using a laser-Doppler velocimeter (ELS-6000, Otsuka Electronics, Japan) in a 10 mmol•dm-3 NaCl solution. The zeta potential for each sample was calculated from the measured value of the electrophoretic mobility. The complete experiment was repeated for a total of three separate assays. The morphology of the sample powders was observed by SEM (JSM-6390LA) at an accelerating voltage of 15 kV. Analysis of the protamine loaded- CPMs powders and protamine sulfate was performed by X-ray photoelectron spectroscopy (ESCaA-5800ci, ULVAC-Phi) using Al K radiation source. The elements in protamine-loaded CPMs powders were detected from the survey spectrum over a range of 0 to 1200 eV. The XPS binding energy values were charge corrected with respected to adventitious carbon at 284.6 eV.
2.4 Evaluation of antimicrobial properties of protamine loadedmicrospheres
Gram-positive bacteria (Staphylococcus aureus; S. aureus, IAM 1011) and Gram- negative bacteria (Escherichia coli; E. coil, K12W3110) were used for the evaluation of antimicrobial activity testing. Culture broth (Nutrient broth from LB, Wako) and culture agar (Nutrient agar from LB, Wako) were used as culturing nutrient sources. Bacteria were grown at 37°C in an incubator.
The CPMs discs incubated with bacteria suspensions for 24 h were also evaluated for cell viability using a BacLight Live/Dead stain kit (Invitrogen, Carlsbad, CA). For this, cell culture medium was aspirated from the well plates containing samples and the samples were rinsed twice with PBS. The Live/Dead stain solution was prepared by mixing equal parts of SYTO® 9 and propidium iodide (PI) and then adding the combined solution to PBS. After adding 0.5 cm3 of the staining solution to each well, samples were incubated for 15 min in the dark at room temperature. Samples were then imaged using fluorescence microscopy (IX71, Olympus, Japan). In addition, the morphology of bacteria at the surface of compacts was observed by SEM.
3. Results and Discussion
Calcium phosphates were prepared via USSP route. XRD analysis showed that the as-prepared (before wash) and washed powders were composed of -TCP and HAp (Figure 1a). The line broadening in these XRD patterns indicated low crystallinity. Figure 1b and 1c illustrate the SEM and TEM micrographs of the washed powders, respectively. SEM micrograph shows that the particles were spherical and their diameters of CPMs were of 0.5-2.0 m. On the other hand, the TEM micrograph reveals that the cores of the spherical particles were translucent. These spherical and hollow particles could be obtained by USSP method regardless of starting solutions [11,12].
Next, to examine the adsorption levels of protamine to CPMs, washed powders were added into various concentrations of protamine solution and incubated for 48 h at room temperature. As a result, the adsorption of protamine to CPMs increased with protamine concentration and reached plateau over 1000 mg•dm-3 (Figure 2a). On the other hand, surface charge is one of the significant factors that affects protein adsorption since it provides electrostatic interactions between the proteins and materials [13]. Therefore, the zeta potential of protamine-loaded CPMs at pH 7.4 was measured (Figure 2b). The zeta potential of CPMs without protamine was about -20 mV. However, the zeta potential of protamine-loaded CPMs increased depending on the protamine concentration and reached maximum at about 18 mV. These results demonstrated that the adsorption of protamine to CPMs was driven by electrostatic interaction following the Langmuir model [14-16]. Furthermore, to investigate the morphological changes by the adsorption of protamine to CPMs, all samples were observed by SEM (Figure 3). SEM micrographs show that all powders were spherical regardless of with/without protamine. However, we could observe needle-like precipitates at the surface of CPMs. These results suggest that -TCP in powders slightly transformed into HAp under reaction with protamine.
Furthermore, to investigate the protamine adsorption onto CPMs, XPS analysis was carried out. Protamine-loaded CPMs with 1000 mg•dm-3 (CPMs (1000)) were examined as a representative sample. On the other hand, CPMs without protamine (CPMs (0)) and protamine sulfate were used for negative and positive control, respectively. XPS survey spectra of protamine, CPMs (0), and CPMs (1000) are shown in Figure 4a. We focused on the N1s photoelectron signal which is the marker of choice for confirming protamine adsorption [17]. We could observe the stronger N1s photoelectron signal in the protamine sulfate powders than that in CPMs (1000). Comparison with CPMs (0) and CPMs (1000) indicated that a weak N1s signal for CPMs (1000) means the adsorption of protamine on CPMs (Figure 4b). These data indicate that protamine mainly adsorbed calcium phosphates via electrostatic interaction. It is possible to predict the adsorption between negative sites of -TCP and HAp (PO4 and OH) and positively charged protamine (NH3) by electrostatic attractions [18].
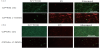
Finally, to evaluate the antimicrobial activity of protamine-loaded CPMs, powders were uniaxially compressed at 50 MPa to form the compacts (CPMs (0) and CPMs (1000)). E. coli and S. aureus were seeded on the CPMs (0) or CPMs (1000) discs and cultured in LB medium for 24 h. At 24 h after plating, the ability of each disc to affect viability of bacteria was tested using Baclight LIVE/DEAD staining kit (Figure 5). Green fluorescing SYTO9 is able to enter all cells and is used for visualization of the living cells, whereas red fluorescing propidium iodide (PI) enters only cells with damaged cytoplasmic membranes. The fluorescence microscope images of E. coli (Figure 5a) and S. aureus (Figure 5b) on the CPMs (0) discs showed that the surface of disc was covered by adhered living bacteria (green). On the other hand, numerous dead E. coli on CPMs (1000) due to destabilization of membrane by protamine were observed (Figure 5a, lower panel). In contrast, there were few fluorescing signals of S. aureus on CPMs (1000) disc (Figure 5b, lower panel). These data demonstrated that protamine-loaded CPMs could prevent bacterial proliferation. The mechanisms of bactericidal activity on gram-negative and grampositive bacteria are still unclear; however, S. aureus would be more sensitive to protamine than E. coli [19]. Furthermore, to examine the microstructure of bacteria on protamine-loaded CPMs discs, the morphology of bacteria was observed by SEM (Figure 6).
The micrographs illustrate that E. coli on the CPMs (0) appeared normal rod-shapes (Figure 6a). However, the morphology of E. coli on the CPMs (1000) disc showed an aberrant shape and formed aggregates (Figure 6b). As for S. aureus, no morphological changes were seen on CPMs (1000) although the number of living bacterial extremely decreased. These results suggest that S. aureus were more sensitive to protamine than E. coli [20]. Taken together, the combination of protamine and calcium phosphates is an efficient approach to prevent bacterial adhesion, prevention and biofilm formation. The immobilization of AMPs onto a biomaterial surface has further advantage and immobilized AMPs may be a promising potential in clinical applications [21].
4. Conclusions
This study showed that protamine loaded-calcium phosphates would be pursued as an alternative strategy for an antimicrobial material for medical devices. Protamine, a cationic antimicrobial peptide, can adsorb to calcium phosphate CPMs following the Langmuir model. The zeta potential of CPMs changed positively by adsorption of protamine dose-dependently. These phenomena were governed mainly by electrostatic interaction between amino groups in protamine and phosphates in CPMs. High concentration of protamine-loaded CPMs particles were positively charged. These molecules could interact with negatively charged bacterial surface and induce bactericidal activity. Previous study showed that an increase in the positive charge of molecules enhanced the inhibition of bacterial growth [7]. In fact, discs fabricated by protamine-loaded CPMs in this study showed antimicrobial activity for both Gram-positive and Gram-negative bacteria. The charge of molecule would be one of the important factors with regard to the antimicrobial effects. In vitro antibacterial evaluation demonstrated that protamine-loaded CPMs appears to be a promising strategy for overcoming resistance to antibiotics. More work is needed to elucidate the mechanism of antimicrobial properties.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
M. Honda designed the study, and wrote the initial draft of the manuscript. Y. Kawanobe and H. Uchida contributed to analysis and interpretation of data. M. Aizawa assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. The final version of the manuscript was approved by all authors.
Acknowledgments
Protamine was a kind gift from Maruha Nichiro Corporation.