1. Introduction
Gestational Diabetes (GD) is defined by the World Health Organization (WHO) as a carbohydrate tolerance disorder, leading to hyperglycemia of varying severity, starting or diagnosed for the first time during pregnancy regardless of treatment necessary and evolution in the postpartum [1-4].
Gestational diabetes is a growing public health problem in view of the evolution of prevalence over the last twenty years, with significant materno-foetal repercussions namely: abortion, in utero death, birth of a macrosomic new born, prematurity, caesarean section [5,6].
The prevalence of GD differs from one country to another, depending on the screening methods, the term and the thresholds used on one hand and the ethnic groups on the other hand [7].
The United Kingdom and the United States have prevalence of 2-6% and 2-10%, respectively [8].
In France the prevalence is estimated between 3-6% [9]. 7-7.6% [8] in Brazil and 13.9% [10] in India.
In Africa this prevalence is 13.9% in Nigeria [11], 7.7% in 2009 and 8.5% in 2011 [12] in Morocco.
In Cameroon, according to a study by Jean Claude MBANYA et al, the prevalence varies from 5 to 17% [13].
In view of the numerous complications of which GD is the cause and the evolution of the prevalence in the world, it is necessary to make an early and precise diagnosis for a fast and efficient management of this affection.
To do this, there are currently two diagnostic methods: the socalled O Sullivan two-step method and the one-time method. The latter is now recommended for the diagnosis of GD by WHO and many societies such as the French National College of Obstetrician Gynaecologists (FNCOG) and the American Diabetes Association (ADA). However, the determination of fructosamine would represent an alternative for the diagnosis of GD. Fructosamine is the level of glycated blood protein, the normal value of which varies with blood glucose. This technique is not unanimous among practitioners as a method of diagnosis.
In view of the above, we have found it useful to carry out a preliminary study in the Department of Obstetrics and Gynaecology of the Douala Laquintinie Hospital in Cameroon to determine the place of fructosamine in the arsenal of the diagnosis of gestational diabetes.
2. Patients, Materials and Methods
Type of study: It was a prospective cross-sectional and descriptive study.
Period, Patients and Methods: It was carried out over a period of five months (from 05th January 2017 to 07th June 2017).
Inclusion and non-inclusion criteria: We included all pregnant women at 24 to 28 weeks of amenorrhoea not known diabetic received in consultation at our health facility during the study period. Pregnant women with fasting glucose (FG) in the first trimester of pregnancy greater than or equal to 1.26 g/L were not included in the study because they were considered to have unrecognized pregestational diabetes; as well as all known diabetic pregnant women, pregnant women taking diabetogenic treatment (thiazide diuretics, beta blockers, corticosteroids), pregnant women beyond the 28th week of amenorrhea.
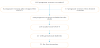
Screening strategy: Fasting glucose (FG) was performed in all pregnant women who were consulted in the department. The diagnosis of gestational diabetes (GD) was retained for these women when this FG was greater than 0.92 g/L twice (but less than 1.26 g/L). Screening consisted of an oral glucose tolerance test with 75g glucose (75g OGTT). The oral hyperglycemia test with 75g glucose (75g OGTT) was performed after a minimum of 12 hours of fasting. It was preferably done between the 24th and 28th week of amenorrhea. This test was performed in pregnant women who had consulted after the first trimester and whose FG in the first trimester was less than 0.92 g/L, but who had classical risk factors of GD. These risk factors were maternal age ≥ 35 years, a history of GD and / or foetal macrosomia, a family history of first-degree type 2 diabetes, and a body mass index ≥ 25 kg/m2. The diagnosis of GD was retained if at least one of the glycaemic values obtained in the OGTT 75g test was higher than the diagnostic thresholds established by the IADPSG 2010, namely 0.92 g/L, 1.80 g/L and 1.53 g/L respectively at H0, H1 and H2 (Table 1). The GD screening strategy used in this study is summarized in Figure 1. Women with OGTT intolerance were excluded from the study.
Parameters studied: The parameters of interest for this study were age, body mass index, history of macrosomia, abortion, in utero foetal death, and glycaemic tests and dosing of fructosamine. Data was collected using a pre-established, pre-tested form.
2.1 Sampling
2.1.1 Sampling technique
Sampling was consecutive and non-exhaustive of pregnant women at 24th to the 28th week of amenorrhea attending prenatal consultations meeting the inclusion criterion.
An informed consent form was signed by the investigators following the explanations they provided, and a research authorization was obtained from the management of the hospital Laquintinie and the institutional ethics committee of the University of Douala.
2.2 Technical procedures
2.3 Dosing of fructosamine
2.3.1 Pre-analytical conditions
Pre-analytical conditions during the dosing of fructosamine:
- Assembly of sampling equipment;
- Preparation and installation of the patient;
- Fasting was not obligatory;
2.4 Collection
The blood sample was collected by venepuncture at the elbow crease and collected on EDTA tube of potassium; then the tube was repeatedly turned to homogenize the whole blood and the anticoagulant.
2.5 Reference method
The reference method used for dosing of fructosamine is High Performance Liquid Chromatography (HPLC).
2.6 Method used
The method used for dosing of fructosamine is the NBT (Nitro Blue tetrazolium) method.
2.6 Principle
Colorimetric test with nitro blue tetrazolium reaction.
The colorimetric test for fructosamine (glycated protein) is based on the ability of keto-amine to reduce nitro blue tetrazolium in an alkaline medium. The formation rate of formazan is directly proportional to the fructosamine concentration and is measured photometrically.
2.7 Quality control and validation of results
The aim was to test the normal and pathological controls before each series of samples, to reassure us that the values obtained comply with the threshold values recommended by the manufacturer. Then our results were validated by a biologist.
2.8 Operating mode
- Aliquot 400μl of blood plasma using a 1000μl pipette into a haemolysis tube.
- Position the tube in one of the wells of Cobas C311 (ROCHE);
- The analysis was done automatically by the automate in 10 minutes.
2.9 Reference values
The reference value is between 205 and 285 μmol/L for non-diabetic adults. A fructosamine concentration above the established expected value indicates the presence of hyperglycaemia less than 1 to 3 weeks old.
2.10 Statistical analysis
All data was recorded and anonymised using Excel software and analysed by SPSS info 16.0 and SPHINXPLUS2 (V5) software.
The Chi 2 test was used for the qualitative variables and the student test for the quantitative variables.
2.11 Ethical considerations
The study was conducted in accordance with the ethical rules related to human research in force in Cameroon and does not involve human experimentation. The tests were conducted using the principles of Good Clinical Practice (GCP) and Good Laboratory Practice (GLP). All precautions were taken to maintain the confidentiality of the data collected from the survey subjects.
No specific risk or inconvenience for the subjects participating in the study is therefore expected. The risks were limited to the time spent conducting interviews. We made every effort to minimize them.
3. Results
During our study period, we recruited 236 pregnant women; Figure 1 summarizes the mode of recruitment; Table 1 describes the glycaemic thresholds required in the oral glucose tolerance test; Table 3 describes the socio-demographic and clinical data of our sample; Table 4 reports the obstetrical history of our pregnant patients, including 11.44% (27 cases) with a history of foetal death in utero, 10.1% abortion (24 cases), 7.63% macrosomia (18 cases).
The 35-45 age group represented 18% of our sample; the average age was 29.25 years with a standard deviation of 5.56 and extremes of 15 and 43 years.
Obesity was found in 6.78% (16 cases) of our sample and 66.53% (157 cases) in our series was overweight.
The average body mass index of our series was 26.44 +/- 2.58 for a minimum of 17.33 and a maximum of 37.89kg/m2 (Table 3).
Our baseline sample was predominantly multi-gravid and multiparous with respectively 137 cases (58.05%) and 128 cases (54.24%). Our gravids were mainly of secondary level of education (50.40%) compared to 38.10% of the higher level (90 cases) and 11.40% of the primary level (27 cases) (Table 4).
Glycaemic tests detected 21 cases of gestational diabetes, including 17 on fasting glucose and 04 on oral glucose tolerance test (OGTT) while their fasting blood glucose was normal (Figure 2).
At the OGTT 11 out of 21 cases had only one pathological value. Glycaemic average was 0.97 g/l at T0, 1.78 g/l at T1, 1.48 g/l at T2 (Figure 3).
This population of diabetic was predominantly multigravid (17 cases: 80.95%), multiparous (16 cases: 76.19%) with 38.10% (8 cases) of history of macrosomia, 28.5% of foetal death in utero (6 cases) and 09.52% history of abortion (2 cases). The 35 years and above accounted for 28.57% of pregnant diabetics; 71.42% (n = 15) were overweight and 9.52% (n = 2) were obese with a prevalence for our study of 8.89% of gestational diabetes at Laquintinie Hospital (Table 3).
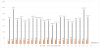
In fructosamine dosing in diabetic pregnant women, 02 of them (or 09.52%), all 29 years old, had high fructosamine levels of 287.6 μmol / L and 290 μmol / L and extremes were 159.30μmol / L and 290μmol / L (Figure 4).
At the ages of 40 and 43 years were low fructosamine levels in the order of 159.3 mmol/l and 177.4 mmol/l respectively with a nonexposing correlation coefficient (r = 0.036). The average rate was 208 85 mmol/l with a standard deviation of 32.29 (Figure 5).
The two 29-year-old diabetic pregnant women with high dosages of fructosamine had different body mass index (BMI) values: 32.55 k/m2 and 23.39 kg/m2 (Figure 5).
On the other hand, the one with the low fructosamine level (159.3 μmol/l) was overweight: 25.82kg/m2.
The correlation coefficient BMI and fructosamine was nonexposing r = 0.13, same for the correlation OGTT and fructosamine (r = 0.42 and p = 0.52 non-significant) in our series (Figures 6-9).
The graph shows the 21 points of fructosamine; the dependence T60 is not significant.
The prevalence of gestational diabetes at Laquintinie Hospital in Douala during our study period was 8.89%.
4. Discussion
Our work aimed to study the interest of fructosamine in the diagnosis of gestational diabetes, after achievement of the OGTT in a population of unknown diabetic women. We recruited 255 pregnant women in the obstetrics and gynaecology department of Laquintinie Hospital in Douala. Two hundred and thirty-six of these women were included in our study and benefited from the OGTT trial and gestational diabetes was diagnosed in 21 pregnant women followed by a fructosamine test.
- Socio-demographic characteristics of pregnant women
-
Clinical data of pregnant women
- Obstetrical history
- Risk factors
The presumptive data for diabetes in pregnancy which are abortions, in utero deaths and macrosomia, were found in our sample in both the baseline and the screened samples, in agreement with the literature [15,16] (Table 3 and Table 4).
The average body mass index was 26.44 kg/m2 superimposed on the 22.2 kg/m2 Touzet et al. [17]. In the diabetic pregnant population, 71.42% (15 cases) were overweight and 9.52% (2 cases) were obese as opposed to Ducarme et al., in whom 23.5% of patients were overweight and 7.5% patients who were obese [18]. This difference is to be attributed to the small number of pregnant women detected in our study. Like Sebire et al. [19], we report that overweight women during pregnancy have an increased risk of gestational diabetes, as well as malformations and macrosomia.
-
Biological data of the diabetic pregnant woman
- Orally induced hyperglycaemia
-
Determination of fructosamine
- Correlation of Maternal age and fructosamine
- Body mass index and fructosamine
We found no correlation between elevated fructosamine concentration and maternal age (r= -0.036). Our results differ from those reported by Frandsen et al. and Robert et al, for whom the fructosamine level in gestational diabetes varies with maternal age. For these authors, the fructosamine concentration tends to increase with maternal age [21,22]. Our data are thus contradictory to those of the literature by the probable fact of our small number of pregnant diabetics.
We did not demonstrate a correlation between fructosamine concentration and overweight (r= 0.13). The literature goes in the same direction with the work of Ardawi et al. in whom the variation of fructosamine level is independent of BMI [23]. So overweight does not influence the variation of the fructosamine level.
- Correlation Glucose / Fructosamine
It means fasting glycaemia for the diabetic pregnant women was 0.97 g/L. In our diabetic pregnancy series, 80.95% (n= 17) had high fasting blood glucose. But 19.05% (n= 4) of diabetics had been diagnosed after OGTT testing. Our results are close to the 26% of Kakad et al. [20] who escaped the diagnosis by the simple measurement of fasting glucose. This justifies the simultaneous realization of fasting glucose and OGTT.
In the series of 21 gestational diabetics, 9.52% (n = 2) had been diagnosed with diabetes after fructosamine dosing. The correlation between fructosamine and oral hyperglycemia was not significant in our study (r= 0.52 and p= 0.48). Our results are consistent with those of Li et al. who found no correlation (r = 0.28) between serum fructosamine levels and oral glucose tolerance test results [24]. This is in line with the work of Uncu et al. which proves that the serum fructosamine assay is not sensitive enough to allow the diagnosis of gestational diabetes [25].
Variations in fructosamine levels during pregnancy are the subject of much controversy. For Staley, the rate of fructosamine is constant during pregnancy, while noting a high degree of interindividual variability [26]. Conversely, other studies show that the level of fructosamine tends to decrease gradually during pregnancy [26,27]. Indeed, the concentration of fructosamine depends on the concentration of albumin; the latter normally decreases during pregnancy because of haemodilution [28]. Thus, the rate of fructosamine tends to decrease during the second trimester and remains low until the end of pregnancy [28]. For this reason, fructosamine cannot be used as a diagnostic test for gestational diabetes.
- Prevalence of Gestational Diabetes
In our basic sample, the 25-35 age group was the majority (44%) corresponding to the usual portion of human procreation with extremes of 15 and 43 years. In pregnant women with diabetes, 57.14% (12 cases) were aged ≥ 35 years, with a maximum age of 43 years. Our data are similar to those of Mimouni (76.6%) [14] and the incidence of gestational diabetes increases with age as reported also by Chan et al. [15]. In agreement with literature data, maternal age is a predisposing factor for the onset of gestational diabetes. The average age of our sample was 29.25 years (Table 3), similar to 29.9 years of Touzet [17].
The prevalence during our study period was 8.89% at Laquintinie Hospital in Douala. Our rate is included in the range of 5-17% reported by Sobngwi et al. [13].
5. Conclusion
Our work aimed to demonstrate the role of fructosamine in the diagnosis of gestational diabetes.
At the end of this work, it was found that women diagnosed with OGTT were not diagnosed with fructosamine, and the increase in fructosamine concentration was related neither to maternal age nor overweight. Moreover, this parameter seems to decrease during the pregnancy because of the haemodilution. It appears that fructosamine is not an ideal marker for the diagnosis of gestational diabetes.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
ESSOME collected the data, designed the manuscript and wrote the article. NDA MEFO’O, EKONO, PENDA, BOTEN,TOCKI, HALLE and FOUMANE read and corrected the article, ADIOGO supervised the writing, corrected the article and validated the definitive version.