1. Background
It is known that numerous spices have medicinal properties and beneficial effects on health [1-3]. Cinnamon leaves and bark are extensively used as spices [4] and as a medicinal herb[3].Recent studies suggest that cinnamon has a positive effect on postprandial glucose metabolism [5-7] and potential beneficial effects such as anti-inflammatory, antibacterial, antifungal, and antioxidant effects [1,5,8]. These effects has been related with their constituents, which could depend on the specie, the plant age, the segment sampling and the extraction method [9]. Several in vitro studies suggested that polyphenolic compounds present in cinnamon can be responsible for insulin enhancing activity in epididymal fat cells and that procyanidin type-A (doubly linked) polymers present in C.burmannii, seems to exhibit insulin-like activity in cells [10-14].
Taking into account the potential health benefits, a large number of clinical trials have been developed to evaluate the effects of cinnamon in humans and revealed beneficial effects on fasting glucose levels [15-22], total cholesterol [15], triglycerides [15], HbA1C [23] and low-density lipoprotein [15,24]. Although several clinical trials have been developed, there are few studies in healthy subjects to investigate the effect of the addition of cinnamon to a high-sugar mealthus, this study was designed to investigate the effect of the ingestion a mousse with cinnamon versus the ingestion of a mousse without cinnamon, on postprandial glycaemia of healthy subjects and also characterize the antioxidant activity of the two meals.
2. Methods
2.1 Setting
This is a human nutrition intervention study with a parallel group design [25] were twenty-four healthy non-diabetic adults with ages between 18 and 35 years were recruited from the local community.
Subjects were randomly assigned in 2 groups (n=12 each): group A (reference meal) and group B (test meal). The participants were required to have an 8 hour fasting before the day of the study and were ask not to ingest any cinnamon at the day before the intervention. For both A and B groups, intervention consisted of the ingestion the reference meal either with or without cinnamon respectively, in the morning (with a duration of proximally 5 minutes). Both interventions were employed under supervision by an investigator.
2.2 Inclusion and Exclusion Criteria
Inclusion criteria included subjects aged 18 or more, both gender with non-diabetic condition (fasting blood glucose level <126 mg/dL) [26]. The exclusion criteria were impaired fasting glucose, pregnancy and lactating or menopausal conditions. Moreover, subjects should not take medication for glycaemia control or antibiotics, nor have gastrointestinal symptoms or diseases. The study also excluded subjects who have altered medication, pregnancy, lactation and allergy to cinnamon.
2.3 Data collection
Following overnight fasting blood glucose level was measured using a capillary drop blood, before intervention (t0). In group A, subjects ingested of 100g of mousse without cinnamon and in group B subjects ingested 100g of mousse with cinnamon. Blood samples were then collected, for each participant, at 30 (t30), 60 (t60), 90 (t90) and 120 (t120) minute after ingestion reference and test meal. Sterilized lancet, glucosemeter equipment and strips for glucosemeter (FreeStyle_Abbott Diabetes Care) were used for blood glucose level measurement. Blood glucose concentrations were measured with the OneTouch Vita® glucometer, which has an accuracy of 98%.
The blood glucose area under the curve (AUC) was calculated above zero and are presented as means. The AUC of each subject was calculated using GRAPH PAD PRISM software (version 5.01).
The anthropometric data was recorded through bio-impedance body fat analyzer InBody® 230 and the height was measured by the stadiometer Craveira Jofre.
No specific diet was imposed and the participants consumed their usual diet. A 24-hours dietary recall was taken preceding each intervention to compare food intake at the day before the intervention between groups. The Food Processor SQL (version 10.5.0) program was used to analyze the food nutritional composition.
All data collection was registered on Excel® file where a coded number was attributed to each participant.
2.4 Ethical approval
This study was approved by a Portuguese state recognized Ethics Committee (Ethics Committee of Cooperativa de Ensino Superior Egas Moniz) and was carried out in accordance with the Helsinki Declaration of 1975 as revised in 2000. A written informed consent was obtained from each participant after given written and oral information about the study.
2.5 Test meal preparation and constitution
The meal consisted in a mousse prepared by the investigator, with the following ingredients and amounts (for a total of 25 doses): 10 gelatin sheets, 1 L of skim milk, 12 egg yolks (204,1 g), 323 g of white sugar, 82,3 g of butter and 963,8 g of sour cream. The test meal consisted of 100 g of mousse mixed with 3 g cinnamon, which was milled with the coffee grinder Taurus Aromatic 150 W. The reference meal consisted of 100 g of the same receipt mousse without cinnamon.
2.6 Chemical analysis (Total phenolic content and antioxidant activity)
Ferric Chloride (III) hexahydrate (FeCl3.6H2O), Folin-Ciocalteu (2,2-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid)), Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), TPTZ 2,4,6-tri(2-piridil)-s-triazine, methanol (CH3OH), nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium (NBT) 2-Amino-2-hydroxymethyl-propane-1,3-diol (tris) and Phenazine methosulfate (PMS) were purchased in Sigma-Aldrich, gallic acid-1- hydrate (C6H2(OH)3COOH.H2O) was purchased in Acros Organics and sodium carbonate (Na2CO3) was purchased in ICS Science group. All reagents were pro analysis grade.
All the absorbance measurements were performed in a Perkin- Elmer Lambda 25. The regents were weight in an analytical balance (Sartorius, ±0.0001g) and all the solutions were done with distilled water.
2.7 Extract preparation
The mousse was subjected to a hydro-ethanolic extract (20:80). Posteriorly, the mixture was filtered using the Whatmanpaper filter. Homogeneous samples were obtained and subjected to chemical analysis.
2.8 Total phenolic content determination
The extracts total phenolic concentration was determined according Folin-Ciocalteu method [4] employing gallic acid as standard. The samples were analysed in triplicate: a volume of 312.5 μl were pipette to an ethanol: water 80: 20 (V/V) solution, to which was added 187.5 μl of water, 5 ml of solution Folin-Ciocalteu reagent (1:10 diluted with water) and 4 ml of aqueous solution Na2CO3 1M. After 15 min the absorbance was measured at 765 nm. Gallic acid was used as the standard solution (Y=0.0034X+0.018 (R2=0.9966)) and the results were expressed in mg of gallic acid equivalents (GAE) / L.
2.9 Characterization of the antioxidant activity: Ferric Reducing Antioxidant Potential (FRAP) assay
This method was adapted from the method described by [27] and is based on the activity of the antioxidants to reduce Fe3+, a colorless ferric complex to a blue-colored ferrous complex, Fe2+ in the presence of 2,4,6-tri(2-piridil)-s-triazine (TPTZ).
The FRAP solution was previously prepared, adding 25 ml of sodium acetate 300 mM pH 3.6,to2.5 ml of 10 mM TPTZ in HCl 40mM, and 2,5ml of FeCl3.6H2O 20 mM. 150 μl of the samples were added with 2850 μl of FRAP solution. The mixture was kept in the dark for 30 minutes and then the absorbance was read at 593 nm. A blank solution was prepared, in which the sample was replaced by 150 μl of water, in the same conditions. The Trolox (6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid), a vitamin E analogous, was used as standard (Y=2,17x10-3X+2,32x10-2 (R2=0,998)) and results were expressed as μM TE.
2.10 Statistical analysis
Statistical analysis was performed with SPSS for Windows (version v19.0; SPSS Institute, Chicago, IL). Data are presented as mean ± SEM. Mann-Whitney U test was used to assess the food intake in the day prior to the intervention between 2 groups. Repeated Measures ANOVA of mixed type was used to assess the postprandial blood glucose level at different times, between 2 groups. Independent sample t-test was used for blood glucose levels area under the curve (AUC) and overnight fasting statistical analysis. All statistical tests were performed at the 5% level of significance.
3. Results
3.1 Sample characterization
General characteristics of sample regarding age, weight (Kg), body mass index (BMI), fat mass (FM), muscular mass (MM), waist to hip ratio (WHR) and waist circumference (WC) are represented in table 1.
SEM: standard error of the mean;BMI: Body mass index; SMM: Skeletal muscle mass; WHR: Waist to hip ratio.
Total calories, macronutrient and dietary fiber intake at the day before the intervention were estimated for both groups. Statistical comparison with Mann-Whitney test revealed homogeneity between groups (p>0.05), table 2.
3.2 Postprandial blood glucose response
The addition of 3 g of C. burmannii result in a slightly increased postprandial blood glucose response at different postprandial times (30, 60, 90 and 120 min), compared with reference meal (without cinnamon), table 3. However, the statistical analysis revealed no interaction between the independent and repeated measures factors (p=0.250), which means that it is not possible to infer about differences in different times.
OGTT: oral glucose tolerance test.
Regarding to the postprandial blood glucose levels AUCs the addition of cinnamon to the mousse slightly increased this value. However, the statistical analysis (Independent sample t-test) revealed that cinnamon addition did not significantly altered the AUC values (p=0.081), table 4.
3.3 Antioxidant characterization of each meal
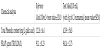
The results of the chemical analysis of the reference meal (mousse without cinnamon) and the test meal (mousse with cinnamon) are represented in Table 5 and revealed that addition of C. burmannii (3g) to the mousse, increased the total phenolic content and the antioxidant activity (assessed by FRAP test).
4. Discussion
The results of the present work suggest that the addition of C. burmannii (3g), to a semi-solid high-sugar desert, slightly increased the postprandial blood glucose (PBG) in healthy subjects, however this result has not statistical significance, compared with PBG after reference meal ingestion. In addition, the blood glucose level AUCs was also not significantly affected by cinnamon addition. These results are in accordance to a cross-over study in healthy subjects [28], where the supplementation of 3 g of cinnamon simultaneously with a highfat meal did not change the postprandial glycemic response. Another study in healthy subjects demonstrated that the addition of 3 g of cinnamon to a low-fat rice pudding test meal had no significant effect on gastric emptying (GE) rate neither in the blood glucose response in terms of the AUCs [20]. This results can be related with cinnamon amount has reported in another work, of the same former authors, were the 6 g cinnamon added to a rice pudding significantly reduced postprandial glycaemia [29]. Moreover, there are other factors that can influence the postprandial blood glucose levels, such as the type of the sample as well as the size, cinnamon specie and types of foods ingested. The results of the present study may also be due to the components of reference meal, which could affect the digestion and absorption of carbohydrate [30]. According with previous studies the high fat content, presented in mousse, could slow down the digestive process and the co-ingestion of lipids and proteins reduce the glycemic response to carbohydrates by delaying gastric emptying and stimulating insulin secretion [30,31]. The control of these factors assumes greater importance in experimental studies, and therefore. Although, the random allocation of participants in groups and the homogeneity between the groups can decrease the interference of individual factors in the results.
Although, cinnamon does not seem to improve postprandial glycaemia in healthy subjects, significantly changes were reported in clinical trials with diabetic subjects [22].
Finally, results from the present study suggest that cinnamon could also increase antioxidant activity of meals. The chemical analysis showed that the mousse with 3 g of cinnamon has 3,5 times higher phenolic content than the mousse without cinnamon. The high total phenolic content is consistent with the values obtained in the antioxidant activity tests, in which the mousse with cinnamon revealed a higher antioxidant activity than the mousse without cinnamon. In the FRAP assay, the values ranged from 91.1 to 941.4 μmol TE/L showing that the total antioxidant activity of the mousse is ten times higher in the presence of cinnamon. These results are in agreement with previous studies revealing that cinnamon contains high levels of phenolic compounds and antioxidant activity [1,32].
5. Conclusion
This study showed that the addition of 3 g of cinnamon to a semisolid high-sugar meal did not significantly reduce the postprandial blood glucose response of healthy subjects, in spite of the higher phenolic content and total antioxidant activity of this meal.
Although the dose of 3 g of cinnamon seems to exhibit a significant reduction in the mean fasting glucose levels in type 2 diabetics, higher doses of cinnamon are apparently required to have an influence in postprandial blood glucose of healthy subjects.
Hence, randomized controlled trials with higher doses of C.burmannii or a different sample, such as type 2 diabetics, are required to clarify the role of this specie in a semi-solid food in the postprandial blood glucose levels.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
Author Contributions
MMM and MAB: conducted chemical research (generation, collection
and analysis of chemical data);
CA: conducted clinical research (generation, collection and first
analysis of clinical data)
CA and MLS: bibliographic research;
MAB and MLS: analysis of clinical data, statistical analysis of the data
and wrote the manuscript;
MMM, MAB and MFM: revision of the manuscript;
MFM and MAB: conception and designed research and had primary
responsibility for the final content;
MAB, CA, MMM, MLS, MFM: read and approved the final
manuscript.
Acknowledgments
The authors declare that they have no competing interests.
Abbreviations
AUC - area under the curve, GAE - gallic acid equivalents, FRAP - Ferric Reducing Antioxidant Potential, BMI - body mass index FM - fat mass, MM - muscular mass, WHR - waist to hip ratio WC - waist circumference, GE - gastric emptying