1. Introduction
In Mongolia, there are so many nomads living in rural area, and feed several animals like Bovine, horse, goat, camel, and sheep. These animal milks and dairy products like AIRAG (fermented horse milk) are very popular in Mongolian people for taking nutrition and keeping their health. To understand the features of these Mongolian animal milks and dairy products, we explored the diversity of bacteria in Mongolian animal milks (Bovine, Horse, Goat, and Camel), and dairy products (AIRAG, Camel milk yogurt, and Biosorbent) by the clone library method of their 16S rRNA genes in previous studies [1]. Then, it was revealed that homologous clones to Lactobacillus helveticus are dominant in the clone libraries of AIRAG and Camel milk yogurt, whereas the other clones to Lactococcus from AIRAG, and Acetobacter and Vogesella from camel milk yogurt. Furthermore, we found that homologous clones to Lactococcus are dominant in Mongolian Bovine, goat, and horse milks, whereas Leuconostoc in camel milk. In Biosorbent, we found homologous clones to Lactobacillus, Lactococcus, Streptococcus and Acetobacter.
In addition, we also found that low molecular weight fractions including peptides (Core, less than 10 kDa) are rich in Mongolian AIRAG and camel milk yogurt. As results, their core fractions exhibited inhibitory activity against serine proteases like trypsin and subtilisin, and it was revealed that they have function as serpin [2]. Then, these results are useful for screening the bioactive lactic acid bacteria strains and the produced peptides from milk and dairy products in Mongolia, and effective products by them.
On the other hand, it also has been investigated that various animal milks contain so many useful and bioactive proteins. Bovine milk components are best studied among these animal milks, and casein is major proteins which occupied about 80% in bovine milk proteins. It is comprised of αs1, αs2, β and κ-caseins, which bind each other and Calcium phosphate, and form micelle particle [3,4]. The rest is whey protein obtained by the acid precipitation of casein from animal milks. The effects of whey protein on human health are of great interest and are currently being investigated as a way of reducing disease risk such as osteoporosis, as well as a possible supplementary treatment for several diseases [5].
Bovine whey fraction contains major proteins such as Lactoferrin (LF), β-lactoglobulin (βLG), and α-lactalbumin (αLA). Lactoferrin is Fe-binding 76 kDa protein (687 amino acids (AA)). LF exhibits antimicrobial activity, antioxidant activity, so that the derivative peptide, Lactoferricin, is a potent antimicrobial peptide which is 42 amino acids digested LF by pepsin [6,7]. Subsequently, β-LG is 18.3 kDa (162AA) protein, which forms monomer at low temperature or acidic condition, and more than dimer on lipid binding [8,9]. βLG belongs to the lipocalin family on the basis of primary structure, which binds to hydrophobic molecules like retinol and palmitic acid. GXW consensus sequence in Bovine βLG is conserved among lipocalin family [10-12] . Moreover, αLA is Ca-containing 14.2 kDa (123AA) protein [13], and α-LA forms multimer complex in the presence of oleic acid, so that it kills some kinds of cells [14-16] . In addition, it was reported that α-LA binds the other fatty acids [17].
On the contrary, the function of βLG and αLA in the other Mongolian animal milks like Goat, Horse and Camel milks except for bovine milk might be poorly understood. Therefore, in order to elucidate their function as lipocalin, we prepared βLG and αLA from Mongolian Goat, Horse, and Camel milks, and investigated their specificity for binding to the fatty acids, lipids, and hydrophobic molecules by fluorescence spectrometry.
2. Materials and Methods
2.1 Materials
Animal milks (Goat, Horse, and Camel milks) used in this study were collected in Ulan Bator, Mongolia at 2011-2012. Fatty acids (Myristic acid, Palmitic acid, Stearic acid, Oleic acid, Linoleic acid), Retinol, 1-aminoanthracene (AMA) were purchased from Sigma-Aldrich. Palmitoleic acid was purchased from Honeywell Fluka, 1-naphthol and Quercetin was from Wako pure chemicals, and 8-anilino-1- naphthalene sulfonic acid (8-ANS) was from MP biomedicals, respectively. Amplicillin sodium salt and Vitamin B1 were purchased from Nakarai tesque. Ultrafiltration unit and membrane (YM10, 10 kDa cut) were purchased from Merck Millipore. Sephacryl S-100 HR was purchased from GE Healthcare Japan. Special grade of all other chemicals were used in this study.
2.2 Preparation of βLG and αLA from Goat, Horse, and Camel milks
Firstly, 100 mL of each Mongolian animal milks (Goat, Horse, and Camel milks) were adjusted to pH 2 by 4M HCl, followed by removed the precipitated casein component by centrifugation (5,000 rpm, 20 min., 4°C). Acid whey fraction was neutralized to pH 7 by 2M NaOH, and each neutral whey fraction was obtained on removing precipitate by centrifugation (8,000 rpm, 40 min., 4°C). Next, each neutral whey fraction was concentrated by ultrafiltration, so that the fraction less than 10kDa was filtered. Finally, each fraction residues (Fr.R, more than 10 kDa) was separated by Sephacryl S-100 gel filtration chromatography. Proteins in the eluted fractions were detected by absorbance at 280 nm, and each purity of βLG and αLA was checked by 15% SDS-PAGE. These purified proteins were dialyzed by 50 mM potassium phosphate buffer (pH 6.5). Then, each protein was named as follows. βLG from goat milk; gLG, βLG from horse milk; hLG, αLA from goat milk; gLA, αLA from horse milk; hLA, αLA from camel milk; cLA, respectively. Each protein concentration of purified βLG and αLA was determined by Bradford method [18], and protein concentration of each sample was adjusted to 0.05 mg/mL for the binding assay as described below.
2.3 Binding assay of gLG, hLG, gLA, hLA, and cLA to the fatty acids and hydrophobic and aromatic molecules
We analyzed the binding properties of each protein (gLG, hLG, gLA, hLA, and cLA) to fatty acids and hydrophobic and aromatic molecules by fluorescence spectra. At first, 10mM stock solutions of fatty acids (myristic acid, palmitic acid, stearic acid, palmitoleic acid, oleic acid, and linoleic acid) and hydrophobic and aromatic molecules (1-naphthol, AMA, quercetin, and retinol) were prepared as dissolved in methanol, whereas those of ampicillin, vitamin B1, and 8-ANS were prepared in 50 mM potassium phosphate buffer (pH 6.5). 6 μL of each stock solution was added to 594 μL of each protein (0.05 mg/mL of gLG, hLG, gLA, hLA, and cLA). Final concentration of fatty acids and hydrophobic and aromatic molecules were set to 100 μM, and according to solvent of each reagent, methanol or 50 mM potassium phosphate buffer (pH 6.5) were used as control, which gave no remarkable effect on protein under these condition. After incubation at 25ºC for 5 min., these Tyr-excited or Trp-excited fluorescence spectra was measured at 25ºC by Hitachi F-2500 fluorometer. These experiments were carried out at least two times individually. Then, Tyr or Trp residues in gLG, hLG, gLA, hLA, and cLA were used as the internal fluorescence probe, and excitation wavelength was set to 275 nm (Tyr) and 295 nm (Trp), whereas emission wavelength was 300- 400 nm (Tyr) and 320-400 nm (Trp), respectively. When the added reagent close to internal Tyr or Trp residues of each protein, Tyrexcited or Trp-excited fluorescence spectra would be quenched [19-25] . The quenching ratio (Q) was calculated by Maximum fluorescence intensity (Fmax) in the presence of the added reagent, as 100% was defined as Fmax in the absence of reagent (added only solvent).
Q (%) = Fmax, reagent / Fmax, solvent ×100
Moreover, final 0-100μM hydrophobic molecules were added to each proteins, followed by measured Tyr-excited fluorescence spectra. Kd values (Binding constant) for each reagent were calculated as the reagent concentration corresponding to 50% of each Q value. Log P values were deposited from MOLBASE (https://www.molbase.com/) by CAS number of above reagents.
3. Results and Discussion
3.1 Preparation of βLG and αLA from Goat, Horse, and Camel milks
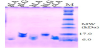
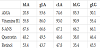
In order to elucidate the function of whey proteins in Mongolian animal milks as lipocalin, we prepared two βLG proteins from goat and horse milks (gLG and hLG), and three αLA proteins from Mongolian Goat, Horse, and Camel milks (gLA, hLA, and cLA), by purification procedure as described above. From 100 mL of each animal milk, we could obtain 1.7 - 4.1 mg of the purified proteins (Table 1) with homogeneity on electrophoresis as shown in Figure 1.
3.2 Binding specificities of gLG, hLG, gLA, hLA, and cLA to the fatty acids and hydrophobic and aromatic molecules
In order to explore the binding properties of each protein (gLG, hLG, gLA, hLA, and cLA) to fatty acids and hydrophobic and aromatic molecules, 13 kinds of final 100 μM reagents were added to proteins, followed by measured Tyr-excited or Trp-excited fluorescence spectra of each protein (Figure 2). Then, on the basis of several reports previously, we selected myristic acid (C14:0), palmitic acid (C16:0), and stearic acid (C18:0) as the saturated fatty acids, whereas palmitoleic acid (C16:1), oleic acid (C18:1), and linoleic acid (C18:2) as the unsaturated fatty acids. In addition, we also chose 7 kinds of hydrophobic and aromatic molecules (ampicillin, vitamin B1, 8-ANS, 1-naphthol, AMA, quercetin, and retinol). As shown in Figure 2, Tyr-excited and Trp-excited fluorescence intensities of cLA decreased in the presence of 100 μM 8-ANS, quercetin, and retinol drastically, whereas slight decrease in case of myristic acid. As to the other proteins (gLG, hLG, gLA, hLA), it was also observed that significant quenching of both fluorescence in the same way as cLA (data not shown). Hence, it was clarified that these whey protein would exhibit the binding properties to hydrophobic molecules like 8-ANS, quercetin, and retinol especially.
Subsequently, we investigated the binding specificities of above 13 reagents to five whey proteins (gLG, hLG, gLA, hLA, and cLA) by using these Tyr-fluorescence spectra (Figure 3), because of showing fully differences in fluorescence intensity between the presence and absence of reagents (Figure 2 (A)). In the binding specificities of three kinds of LA (gLA, hLA, and cLA) to fatty acids, it was observed that slight decrease of Tyr fluorescence intensities (Q values) in the presence of saturated fatty acids, as compared with those in unsaturated fatty acids (Figure 3 (A)-(C)). Then, hLA shows affinity to the palmitic acid (Q value: 86.1%), whereas gLA and cLA might prefer to the myristic acid (74.5% and 86.5% respectively). As to the binding specificities of hydrophobic molecules to three LAs, it was found that they exhibited the decrease tendency on Q values (less than 70%) in the order of 1-naphthol, vitamin B1, AMA, quercetin or retinol, 8-ANS, though ampicillin was no significant effect on these LAs in spite of being an aromatic ring. Especially, it was revealed that three LAs have high binding affinities for quercetin, retinol, and 8-ANS, because Q values of three LAs for them decreased remarkably (less than 20%).
On the other hand, in case of two kinds of LG (gLG and hLG) to fatty acids, hLG showed slightly decrease in Q values on adding to fatty acids (85-90%), so that it has broad affinity for fatty acids (Figure 3 (D)), whereas gLG exhibited weak affinity for the only saturated fatty acids (89-93%, Figure 3 (E)). In addition, they showed the decrease on Q values (less than 70%) in the order of ampicillin (only hLG), 1-naphthol, vitamin B1, AMA, quercetin or retinol, 8-ANS, especially Q values of quercetin, retinol, and 8-ANS decreased less than 10%. Therefore, it was elucidated that two LGs also have high binding affinities for quercetin, retinol, and 8-ANS, and their specificities are similar to three LAs as described above.
3.3 Concentration dependency of gLG, hLG, gLA, hLA, and cLA on binding to aromatic and hydrophobic molecules
As mentioned above, five whey proteins (gLG, hLG, gLA, hLA, and cLA) exhibited high binding affinities on hydrophobic and aromatic molecules like 1-naphthol, vitamin B1, AMA, quercetin, retinol, and 8-ANS. Furthermore, we explored concentration dependency of gLG, hLG, gLA, hLA, and cLA on binding to these aromatic and hydrophobic molecules (vitamin B1, AMA, quercetin, retinol, and 8-ANS), which were less than 50% of Q values on adding 100 μM reagents (Figure 3). Final 0-100μM vitamin B1, AMA, quercetin, retinol, and 8-ANS were added to each proteins, and then, Q values were calculated by measured Tyr-excited fluorescence spectra (Figure 4).
As shown in Figure 4, Q values for all proteins decreased as added reagent concentration increased, and it was found that Tyr-excited fluorescence quenching had concentration dependency. In hLA (Figure 4 (A)), the addition to AMA and vitamin B1 induced significant decrease to 33.1% and 50.9% of Q values even at 33 μM reagent, whereas the others decreased gradually. Hence, it was proved that AMA might be the most suitable for binding to hLA. As to gLA (Figure 4 (B)), vitamin B1 exhibited low affinity for gLA, though AMA also showed the highest affinity of all reagent for gLA as same as hLA. In contrast, it was observed that cLA had low affinities for vitamin B1 and AMA, whereas quercetin and retinol showed high affinities for cLA as same as gLA (Figure 4 (C)). Therefore, it was proved that three LAs had different specificity for these aromatic and hydrophobic molecules.
Subsequently, hLG exhibited high specificity for quercetin and retinol as compared to AMA and vitamin B1 (Figigure 4 (D)). Significant fluorescence quenching was not observed even at 66 μM AMA and vitamin B1 (86.0% and 95.0% of Q values). On the contrary, gLG showed high affinities for AMA and vitamin B1 at 33 μM reagent (37.8% and 53.8% of Q values), whereas drastic decrease of Q values was observed on adding more than 66 μM quercetin (23.8%) and retinol (24.4%) to gLG (Figure 4 (E)). Then, it was revealed that hLG and gLG would bind to quercetin and retinol at more than 66 μM concentration, and gLG also binds to AMA and vitamin B1 even at 33 μM concentration.
On the basis of these results, we evaluated Kd values (Binding constant) of five whey proteins Kd values for vitamin B1, AMA, quercetin, retinol, and 8-ANS, as the reagent concentration corresponding to 50% of each Q value (Table 2). In hLA, AMA was the lowest Kd value of them (20.8 μM), followed by quercetin, retinol, Vitamin B1, and 8-ANS. In contrast, Kd values of gLA and cLA for AMA (53.6 μM and 74.6 μM) and vitamin B1 (86.0 μM and 93.0 μM) were higher than hLA, though they showed similar affinities for quercetin (46.6-49.5 μM), retinol (43.7-53.4 μM), and 8-ANS (66.6- 67.6 μM). Therefore, it was proved that three LAs might bind to quercetin, retinol, and 8-ANS in similar way, and had different specificities for binding to AMA or vitamin B1 among them.
As to hLG, quercetin and retinol exhibited low Kd values (38.0 μM and 35.4 μM), followed by 8-ANS, AMA, and Vitamin B1. On the other hand, gLG indicated the lowest Kd value of for AMA (30.1 μM), and also showed smililar affinities to hLG for quercetin (46.4 μM), retinol (45.5 μM), and 8-ANS (66.1 μM). Moreover, gLG would also bind to Vitamin B1 with 55.4 μM of Kd value. Hence, it was implied that both two LGs might bind to quercetin, retinol, and 8-ANS in similar way, and only gLG had high specificities for binding to AMA and vitamin B1.
Furthermore, we explored for the relationships between these Kd values and logP of aromatic and hydrophobic reagents (vitamin B1, AMA, quercetin, retinol, and 8-ANS). LogP values exhibit a degree of hydrohobicity/hydrophilicity, so that the retinol is the most hydrophobic among them (logP: 5.51), and logP decreased in order of 8-ANS (5.09), AMA (4.16), quercetin (1.99), and vitamin B1 (1.19). As compared to Kd values in Table 2, minimal Kd was found for AMA in hLA and gLG (20.8 μM and 30.1 μM), whereas the others implied approximately negative correlation between Kd and logP. From these considerations, we deduced that the hydrophobicity like AMA might be the most suitable for ligand-binding site of hLA and gLG, and it may also affect on binding of hLG, gLA, and cLA to hydrophobic molecule.
Additionally, we assumed that the differences in binding properties of three LAs and two LGs might be derived by homology among their amino acids sequences. Three LAs are comprised of 123 amino acids as same as bovine LA (homology in amino acids: 68-95%) [26-30] . Bovine LA, hLA, and gLA contain four Trp (Trp26, Trp60, Trp104, Trp118) and four Tyr residues (Tyr18, Tyr36, Tyr50, Tyr103), whereas cLA contains five Trp (above four Trp residues and additional Trp123) and three Tyr residues (Tyr36, Tyr50, Tyr103). In Figure 2, we confirmed specific quenching of both Tyr and Trp fluorescences in cLA by addition to quercetin, retinol, and 8-ANS, like similar behavior observed in hLA and gLA. Hence, we deduced that common three Tyr and four Trp residues may be crucial to bind to quercetin, retinol, and 8-ANS, whereas lacking of Tyr18 and addition of Trp123 in cLA may contribute to the differences in binding properties of the other fatty acids and aromatic and hydrophobic molecules between cLA and the other LAs. Although LAs are not belonged to lipocalin family, it was elucidated that three LAs may have lipocalin-like function from these results.
On the other hand, two LGs are consist of 162 amino acids like bovine LG [31-37] . Bovine LG and gLG (homology in amino acids: 96%) contain four Tyr (Tyr20, Tyr42, Tyr99, Tyr102) and two Trp residues (Trp19, Trp61), whereas hLG has five Tyr residues (Tyr42, Tyr86, Tyr99, Tyr102, Tyr121) and sole Trp19, which was conserved in lipocalin family [10-12] . As exemplified in Fig. 4 and Table 2, hLG exhibited low affinity for AMA and vitamin B1, in contrast with high affinity of gLG for them. Then, we considered that conserved Tyr42, Tr99, Tyr102, and Trp19 play a key role in binding to quercetin, retinol, and 8-ANS as lipocalin [10,11], so that variations of Tyr86 and Tyr121, and lacking of Tyr20 and Trp61 might cause their differences in affinity for AMA and vitamin B1.
From these results, it was clarified that these whey proteins have specific binding to hydrophobic and aromatic molecules with polar group(s) partially. Therefore, it was deduced that these whey proteins might function as lipocalin, and transport hydrophobic molecules in Mongolian animal milks. Whey protein like LA and LG has possibilities to show affinity for various hydrophobic molecules like cholesterol or lipids, or aromatic molecules like nucleotide and its derivatives. Further studies must be needed, and our exploration for binding features of whey proteins to various molecules is now undertaken.
4. Conclusion
In this study, in order to evaluate their binding properties of five whey proteins (gLG, hLG, gLA, hLA, and cLA) as lipocalin, we prepared them from Mongolian animal milks (Goat, Horse, and Camel milks), and measured their fluorescence spectra with or without six fatty acids and seven hydrophobic molecules, by fluorescence spectrometry using internal Tyr residues as probe.
As results, we found that each Tyr-excited fluorescence spectra was significantly quenched by adding 100μM 1-Naphthol, Vitamin B1, AMA, Quercetin, Retinol, and 8-ANS, whereas slight decrease of fluorescence intensity was observed when fatty acids were added. In particular, it was observed that their fluorescence spectra were quenched and maximum wavelength shifted remarkably when added Quercetin, Retinol, and 8-ANS. Furthermore, we evaluated Kd values of five whey proteins for Vitamin B1, AMA, Quercetin, Retinol, and 8-ANS, and then it was revealed that these whey proteins have specific binding properties to the hydrophobic and aromatic molecules with the polar group(s) partially. Therefore, these whey proteins might function as lipocalin and transport hydrophobic molecules in Mongolian animal milks.
Competing Interests
The authors declare that they have no competing interests.