1. Background
Ready-to-Use Foods (RUFs) play a vital role in the treatment and/ or prevention of undernutrition in vulnerable individuals such as young children, and adults with compromised health status. RUFs are broadly grouped as therapeutic or supplementary (RUTF or RUSF, respectively) [1]. RUFs, as their name indicate, can be directly consumed from their packages without requiring preparation or trained personnel to administer them. Nutritionally, RUFs are comparable with Formula-75 (F-75) and Formula-100 (F-100) [2], that are milk-based therapeutic foods to rehabilitate children with severe acute malnutrition (SAM) treated in hospitals or feeding centres. RUTFs are primarily used in the nutritional rehabilitation of children with SAM whereas RUSFs are meant to treat children with moderate acute malnutrition (MAM) [3]. RUSF also refers to a medium-quantity or large-quantity lipid-based nutrient supplement (MQ/LQ-LNS) used to treat/supplement the nutrition of adults with compromised health status (such as those with HIV), and to a small-quantity lipid-based nutrient supplement (SQ-LNS) used to supplement the diet of children being given complementary foods but needing an improved diet [4].
Undernutrition can be acute or chronic in form. Chronic undernutrition results from a long term or recurrent inadequate diet and/or other co-morbidities (such as infectious diseases). In children, chronic undernutrition manifests itself in a linear growth deficit, known as stunting, and is expressed as height-for-age z score (HAZ) below -2 from the median height of WHO reference population. HAZ score below -3 from the WHO growth standard defines severe stunting [5]. In contrast, acute malnutrition arises from a short-term nutritional inadequacy due to transitory food insecurity or other emergency situations such as famine and drought. Acute malnutrition is expressed as low weight-for-height z score (WHZ) below -2 from the median weight of WHO reference population or a mid-upper arm circumference of <11.5cm for SAM and 11.5cm-12.5cm for MAM cases [6]. WHZ score between -2 and -3 on the WHO growth standard defines moderate wasting whereas WHZ score <-3 from the WHO growth standard defines severe wasting [5]. Globally, 33 million children are moderately and 17 million severely wasted [7,8]. If untreated, severe wasting increases the risks of mortality and morbidity significantly (by nine-fold), resulting in a million deaths among children annually [6,9].
Ethiopia continues to carry a significant burden of acute malnutrition. According to the 2016 Demographic and Health Survey (DHS), 10% of all children under the age of 5years are wasted and 3% are severely wasted [10]. The DHS occurs every 5 years and the proportion of wasted children, unlike stunting, has not declined meaningfully since the first DHS in Ethiopia in 2000. Stunting has declined by about six percentage points every five years in the last 15 years although there still remains high burden of child stunting (38%) in the country. Moderate wasting, i.e. MAM, is twice the prevalence (7%) as severe wasting, i.e. SAM (3%) in Ethiopia [10]. Children with MAM not only have greater risk of mortality but also could progress to SAM (and increased risk of mortality) in the absence of adequate nutritional support [11]. Hence addressing MAM in the affected children through appropriate nutrition intervention prevents the mortality.
Traditionally, children with severe acute malnutrition were treated in an inpatient setting in therapeutic feeding centres (TFC), hospitals or other health facilities where F-75 or F-100 are used as dietary therapy until the children are stabilized and their appetite and tolerance for regular food is regained [2]. These special formula diets need to be administered by trained health personnel as they require sterile preparation. Due to the limited availability of health facilities and trained personnel, coverage and effectiveness of this treatment regimen is limited. However, with the innovation of Ready-to-Use Therapeutic Foods, World Health Organization (WHO) along with other UN agencies endorsed the Community-based Therapeutic Care (CTC) as the preferred treatment model, as opposed the conventional inpatient care [9]. RUFs and their use in outpatient setting has resulted in increases in coverage of SAM and MAM treatment, mainly because affected children with no medical complications but with appetite, can now be treated in their homes and communities. RUFs can be used where there is limited access to clean water and they have prolonged shelf-life because of their very low moisture content [1,12-14].
Over the last decade, Ethiopia has used RUTF/RUSF in the treatment of children with SAM or MAM, and lately HIV infected adults initiating antiretroviral treatment. However, the overall experience of RUF use in Ethiopia has not been explored. This paper aims to provide a scoping review of all published RUTF and RUSF studies in Ethiopia we retrieved using specific search terms and reflect on observed opportunities and challenges that have important implications for future programs engaging in formulations, production and delivery of RUTF and RUSF for treatment and prevention of all forms of malnutrition in Ethiopia.
2. Methods
2.1 Criteria for inclusion
This scoping review included all studies found with a direct or indirect reference to RUTF or RUSF or LNS products and their use in the treatment of malnourished children or other targets, as well as in supplementing diets of groups to prevent under nutrition in Ethiopia. We included all Ethiopia focused RUF studies our search retrieved and published up to September 2018, with no regards to the size or design of the studies.
2.2 Search terms and database
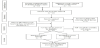
Key search terms used include “Ready-to-Use Therapeutic Food” or “RUTF”, Ready-to-Use Supplementary Food” or “RUSF” or “Lipidbased Nutrient Supplements” or “LNS” AND “Ethiopia”. Databases searched include Ovid MEDLINE, PUBMED, and GOOGLE. MEDLINE search was not used as MeSH (Medical Subject Headings) would not include the search terms and the articles generated were not relevant to the topic. We also located one article through hand-searching and reference checking. One article was excluded from PUBMED search after reviewing the title and abstract. We restricted our search for articles in English language published up to September 2018. The earliest article retrieved was from 2006 which coincides with the time WHO and other UN agencies issued a joint statement endorsing the community-based management of severe acute malnutrition with the innovation of RUTF [9]. Figure1 shows a summary of the search process and the literature retrieved from each database as well as the screening process. The flow chat has been adapted from the PRISMA publication [15].
2.3 Analysis and presentation of data
The full length of each article was reviewed to extract relevant information for the current review. Emphasis was given to identifying the main objectives of the studies, design, target/study population, type of ready-to-use food involved and key findings. Summary information on these key elements has been presented in Tables 1 and 2 (Supplementary File). A column was included to note some key remarks and conclusions from each study. Some studies involved multiple countries including Ethiopia; however, we presented only the information pertinent to Ethiopia. Overall, the studies have been organized in two main categories arranged chronologically by year of publication: Table 1 presents summary of all studies dealing with ready-to-use therapeutic foods (RUTF) whereas Table 2 presents summary of ready-to-use supplementary foods (RUSF). RUSF also include those supplementary or complementary food products known as small quantity or large quantity lipid-based nutrient supplements (SQ-LNS and LQ-LNS, respectively). The studies in each table are further grouped based on whether they deal with HIV or non-HIV infected population.
3. Results
3.1 General description of the studies
A total of 23 original research articles published up to September 2018 were considered for the current review. Most articles (19 of the 23) dealt with the use of RUTF (9/19) or RUSF (9/19) to treat acute cases of undernutrition or supplement existing diet to prevent chronic undernutrition in children or adults in Ethiopia. One study assessed adherence to the nutrition support program among HIV positive patients receiving both RUTF and RUSF [16]. Another study [17] examined supply chain related factors that affected community level availability of medicinal supplies, including RUTF, used in the treatment of common childhood illnesses. The remaining 3/23 articles dealt with the formulation and acceptability of novel RUTF [18,19] or RUSF [20] products from local ingredients.
Five of the 23 studies had a qualitative (n=3) or mixed methods (n=2) approach to explore issues surrounding the use of RUTF or RUSF while the remaining 18 followed a quantitative approach to assess and evaluate effectiveness of programs in the treatment of acute malnutrition. Fifteen of the 23 studies involved SAM (n=11) or MAM (n=4) children in the age group of 6-59 months (or their caregivers and/or community health workers), while seven studies dealt with HIV infected adults participating in antiretroviral treatment (ART) and nutrition support programs.
4. Use of Ready-to-Use Therapeutic Foods (RUTF) in Ethiopia
Fourteen studies involved RUTF use. Of these, 12 dealt with non- HIV population while 2 studies dealt with HIV positive population (Table 1). Fourteen studies involved the use of RUTF product in the treatment of children < 5 years suffering from SAM, and in nine studies the RUTF product was Plumpy’Nut®. Another three studies did not specify the RUTF product involved, but Plumpy’Nut® would be the likely product [17,21,22]. The remaining 2/14 described novel RUTF product formulations from local ingredients [18,19].
Studies of Plumpy’Nut® rollout and use: Five qualitative or mixed method studies examined introduction and continued use of Plumpy’Nut® [16,22-25]. After the 2007 endorsement by joint UN agencies of the community-based management of SAM using RUTF products [9], researchers took interest in evaluating the effectiveness and cost of managing SAM with RUTF, perceptions around RUTF, barriers to access SAM services, and poor adherence to therapeutic programs in Outpatient therapeutic Program (OTP) settings. The earliest study of RUF use in Ethiopia examined the experience of rolling out OTP services for treatment of SAM from hospital to the community level health posts [23]. This study found that OTP for management of SAM at community level worked well with the use of Plumpy’Nut® but also noted supply related challenges with the RUTF product. A study by Tekeste et al. [22] considered cost related issues around treatment of SAM with RUTF in CTC versus in TFC. The findings showed cure rates were high at ~95% in both treatment models but treatment cost/child in CTC ($135) was less costly as inpatient care ($285)[22]. A study conducted in 2014 found distance, high opportunity costs, child refusal of the RUTF, and poor knowledge of available services as key barriers to access SAM treatment services in Ethiopia [24]. A similar study explored factors influencing adherence to nutrition support program among HIV positive adults in a facility-based study and found low adherence due to disliking the taste of the RUF, missing follow-up appointments, sigma and sharing of the RUTF [16]. This study also found that low level of education, poor knowledge about benefits of RUF, longer program duration, consumption of >2sachets/day and not being informed about the length of the program, as factors negatively influencing adherence. A study by Tadesse et al. [25] assessed caregivers’ perceptions toward RUTF (Plumpy’nut®) use to treat SAM. Overall, Plumpy’nut® was perceived as effective, but was also found to be a food for other family members, and at times, a commodity for sale.
Effectiveness of RUTF use in SAM children and HIV children and Adults: Seven studies followed a quantitative approach to assess effectiveness of the SAM treatment using RUTF products in OTP settings [17,21,26-30]. Chaiken et al. [27] wrote in 2006 an early examination of RUTF effectiveness in CTC versus conventional approach to treating SAM in South Ethiopia. They found that the former resulted in comparable recovery rates from SAM but with far higher coverage rates compared to the latter. Several studies assessed treatment outcomes [21,28,30] and factors affecting time to recovery [29] of SAM children treated with Plumpy’nut® in OTP setting in Ethiopia. Each of these studies found significant positive contribution of RUTF in recovery of SAM children but noted supply related and operational challenges such as inadequate supply of products and poor capacity of service providers. Yebyo et al. [30] reported below minimum (<75%) recovery rate of SAM children in north Ethiopia and attributed the cause to poor capacity of OTP service providers. Another study identified challenges on the side of the beneficiaries (unintended use of RUTF) and OTP providers (inadequate supply of RUTF and use of inappropriate exit criteria) [21]. Limitation with RUTF product availability in Ethiopia was also noted by Chandani et al. [17] while examining supply chain factors affecting basic medicines to treat childhood illness. Mengesha et al. [29] identified the type of malnutrition (i.e., kwashiorkor or marasmus), older age, lower rate of MUAC gain and lower admission weight as factors affecting recovery time of SAM children in south Ethiopia. A similar study in south Ethiopia also found below standard recovery rates of SAM and recommended strengthening capacity of OTP providers [28]. Bhagavathula et al. [26] reported on lower than expected recovery rates of HIV infected SAM or MAM children or adults in Gondar Hospital and speculated possible food sharing or the selling of RUTF among adults as possible cause.
Novel RUTF formulations from local ingredients: A study by Ryan et al. [18] used linear programming tool to create novel RUTF based on local ingredients but of equivalent nutrient and energy profile as the standard RUTF. The study signaled potential for local production of RUTF by producing 32 new formulations the final ingredients of which included fish, dairy powder and seeds, grains and legumes. Later, acceptability of some of the new formulations were assessed by the same group. Children similarly preferred and tolerated the new formulations as the standard Plumpy’Nut® [19]. The study noted that alternative RUTF could be developed at 60% of the cost for standard RUTF.
5. Ready-to-Use Supplementary Foods (RUSF) in Ethiopia
A total of nine articles were retrieved dealing with RUSF spanning the period 2006 - September 2018. Table 2 summarizes the nine studies as those dealing with non-HIV population and HIV positive patients. The studies involved various RUSF products and covered a range of issues surrounding their use both for MAM cases of children and HIV infected adults as well as use in the complementary feeding of children.
RUSF in MAM and complementary feeding children: Three studies are summarized in this group [31-33]. One study compared recovery rates of MAM children participating in supplementary feeding programs that used RUSF (Supplementary Plumpy; Nutriset) and Corn-Soy Blend (CSB), fortified blended flour [34]. The result indicated better recovery rate in the RUSF group (15% more) than the CSB group despite larger ration size in the CSB group [31]. A follow up study showed existence of significant sharing of CSB flour with other family members compared to the RUSF product (Plumpy’Sup) [32]. The study suggested strengthening the nutrition education component of supplementary feeding program to ensure RUSF are used only for intended target. The third RUSF study assessed willingness to pay (WTP) for 1 week’s supply of Nutributter® (RUSF) by parents of complementary feeding children in urban settings [33]. The finding showed high “stated” WTP (96%) which did not much with “actual” WTP during simulated market experiment. The study also noted that “stated” WTP may not be predictive of “actual” WTP.
RUSF in HIV infected adults: Of the nine RUSF studies, five dealt with use in HIV infected adults participating in ART [35-39]. The study by Olsen et al. [37] explored the use; perceptions and acceptability of RUSF (Plumpy’S upTM) by HIV infected adults initiating ART in Jimma area, southwest Ethiopia. Findings showed the product was well accepted-thought as “nutritious” and protective from the negative side effects of ART drugs. However, the risk of HIV status disclosure and its social consequences was raised as main concern. The authors suggested that such nutrition support programs should consider social contexts to minimize unintended consequences to beneficiaries due to their use of RUSF. The remaining four studies evaluated the effect of lipid-based nutrient supplements (LNS) containing whey/soy protein, and the timing of the supplementation, on various outcomes among HIV positive adults. One study found beneficial effects on weight gain, grip strength and immune function [36]; another study [35] found an interaction between LNS consumption and plasma concentration of components of the ART drugs but also noted that the “clinical relevance” of the interaction was not clear. This study also reported a 28% poor adherence (i.e., <75% consumption of the daily LNS ration) as well as some dropouts (22/282) because participants disliked the product. The third study showed better quality of life among those HIV positive adults who received LNS in the first three months of ART compared to those who did not receive LNS in early stages [38]. The fourth study compared level of serum 25-hydroxyvitamin D (25OHD) status among HIV positive adults who received LNS containing vitamin D3 or no supplementation during the first three months in ART programs [39]. The result showed LNS supplemented group had increased levels while the no-supplement group had decreased levels of serum 25OHD, indicating the need to replenish vitamin D levels to prevent reduction during ART.
Local production of RUSF: Only one study dealt with a locally produced RUSF product and its potential use in the treatment of MAM children in Ethiopia. This single study assessed sensory acceptability of four chickpea-based RUSF formulations in various regions of Ethiopia [20]. The study found an overall higher rating for “chickpea-only” product by mothers of the children. It also noted that older children (48-59 months) ate more of the product compared to younger children (6-11 months). The study suggested that World Food Program’s Purchase for Progress program could utilize this opportunity to promote chickpea production for market.
6. Discussion
The problem of malnutrition has been a significant concern in Ethiopia for many years due to chronic and transitory food insecurity and other emergency situations such drought and famine. Recurrent drought, erratic rainfall patterns, small farm-holdings, as well as poor farming practices have limited crop yield in Ethiopia and, thus, causing segments of the population to experience chronic and transitory food insecurity [40]. Vulnerable households, particularity those with young children, suffer associated nutritional consequences of food insecurity, including SAM and MAM. Children with severe acute malnutrition are at greater risk of dying compared to those with WHZ > -1 [6,41]. The importance of life saving nutrition interventions to affected children cannot be overemphasized. Although several studies in Ethiopia reported on use and effectiveness of RUTF and RUSF in the management of malnutrition, available evidence on RUTF and RUSF in Ethiopia published within the last 10 years shows many opportunities and challenges to RUF use.
One of the opportunities observed for RUF use in Ethiopia is a growing number of target groups. Initially, RUTFs were used to treat children with SAM [27]. Then, RUF use included the treatment of MAM children [31]. However, research reporting on the type, use and effect of RUFs in the treatment of MAM in Ethiopia are limited, warranting the need for more research. Likewise, RUF use has found a role in nutrition support programs for HIV infected adults [16,35,37] or children [26] participating in ART programs. There is also a move to introduce SQ-LNS in the diet of young children (<2 years) to prevent chronic undernutrition [33]. The use of LNS products to prevent undernutrition or promote linear growth in young children has been reported in other developing countries such as Mali [42] and in Democratic Republic of Congo [43]. Besides the growing target group for RUF use, initiatives have been observed to formulate novel RUFs products from local ingredients [18-20]. Similar attempts have been reported in other countries such as India, Pakistan and Ghana [19]. The novel RUFs formulation effort has been argued to cut down cost of standard RUFs while increasing coverage and its economic sustainability. Another observed trend is the diversity in the types of RUF products available. Products range from the standard therapeutic foods, to supplementary, and complementary foods that are packaged in small, medium or large quantity-this facilitates the targeting of various groups and enhances program effectiveness.
Various challenges associated RUFs use in Ethiopia, particularly among HIV infected adults, were observed. The sharing of RUF products with other family members and/or selling as market commodity, or disliking the taste of the product were significant problems contributing to poor adherence [16]. Similar studies in Malawi [44] and Kenya [45] raised the issue of poor acceptance of the RUTF products by HIV infected adults-partly because the products are less adapted to adults as they were originally made for young children-and suggested the need to develop products more suited for adult taste. However, a study in Haiti showed high acceptability of RUTF products among HIV infected adults compared to Corn-Soy Blend (CSB) [46]. The study by Karakochuk et al. [32] reported the existence of significant food sharing practice of RUFs and CSB flour among family members after observing lower recovery rates in RUSF and CSB fed children with MAM in an earlier study [31]. The studies have noted that the practice of sharing was greater among those in the CSB flour than RUSF group. These unintended uses of RUFs were also reported elsewhere in Ethiopia [21,25] and Haiti [46]. Sharing of RUF has been reported in studies in Malawi and Niger where mothers shared foods intended for young children with other family members [47,48].Since RUFs are packaged based on the nutritional needs of targeted children, the unintended use (such as sharing and selling) of these product decreases the quantity available for the intended child, leading to increased risk of morbidity and mortality. Unlike the CSB, studies have noted that RUF were shared mostly among children <5y within the same family [32,48]. Hence, emergency nutrition and prevention programs should account for potential sharing of RUFs while assigning ration size and strengthen nutrition education component of such programs to increase effectiveness.
High cost associated with RUF products-mainly due to the cost of powdered milk included as ingredient, was another key concern. The high cost of standard RUFs put the economic sustainability of the products into question as the places most needing the products are low-income countries. This has triggered the need to explore alternative products, such as those that use local ingredients. Ethiopia based studies on this issue are limited although evidence exists for the technological feasibility of local production of RUFs [13]. The need to cut down cost by considering new formulations of RUFs, such as those replacing costly dairy ingredient in standard RUFs, have been previously suggested [49]. However, as shown in a Malawi case study, replacing the dairy ingredient in the standard RUFs with non-dairy alternative may not be as effective [49,50]. More research is needed to complement the limited evidence on the issue of local RUFs production and effectiveness of such products in Ethiopia.
The issue of stigma associated with RUFs use among HIV infected adults was raised as a barrier to service utilization and poor adherence to nutrition support programs [16,37]. Strengthening nutrition support programs, patient counseling as well as innovative ways to minimize the association of RUFs use and HIV status, such as developing similar products for a non-HIV population, should be sought to mitigate the stigma.
Lastly, studies dealing with the use of LNS products in the prevention of chronic undernutrition and the promotion of linear growth are very limited or non-existent in Ethiopia. The exception is the single study that explored market demand and parents’ willingness to pay for Nutributter® in urban Ethiopia [33]. Research results from other developing countries, such as Malawi, Ghana, Bruikna Faso, Chad and Haiti, were inconclusive on benefits of LNS to linear growth [51,52]. Hence, further research is needed to understand the effect of LNS in prevention of chronic undernutrition in Ethiopia.
A limitation of our scoping review was lack of searching the gray literature as reports on RUTFs may be produced but not published in journals; hence this review by no means is exhaustive. For example, Ready-to-Use Complementary Food is a new initiative not yet described in Ethiopia [4].
The studies reviewed touched on a range of areas, including effectiveness of RUTF/RUSF in the treatment SAM or MAM (mainly in community-based therapeutic care), local production of RUF, perceptions toward RUF by beneficiaries, product cost and challenges associated with the implementation of SAM/MAM care in the outpatient setting. We also noted an increasing trend in diversity of the target groups and RUF products over the last decade.
7. Conclusions
The current review has shown that Ready-to-Use Foods have been integral part of the management of acute malnutrition in Ethiopia, particularly among children 6-59 months of age. The study also showed a growing interest in the use of RUFs including lipid-based nutrient supplements among HIV positive adults. Several of the studies reviewed primarily explored effectiveness of SAM treatment in OTP settings. Some studies have explored cost of RUF products, barriers to access, perceptions and experiences of beneficiaries and program implementers surrounding RUFs and their use in therapeutic and supplementary feeding programs. Small numbers of studies have also considered potentials for production of novel RUTF or RUSF from local ingredients. Overall, an increase in diversity of target groups for RUFs products, diversity in products in kind and packaging of the products themselves, along with a potential for a cost-effective local production, has been observed. Food sharing, trading of RUFs as commodity, high cost of standard RUFs, as well as stigma associated with RUF use and disliking the taste of RUFs were some of the key challenges observed warranting the attention of nutrition support program providers and product developers.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
C.H. and S.W. conceived of the idea; G.E. and S.W. designed and conducted the review of literature; G.E. drafted the manuscript; S.W. and C.H. critically reviewed the manuscript. All authors gave final approval.
Acknowledgments
We would like to thanks Global Institute for Food Security, University of Saskatchewan for financial support.
Abbreviations
ART: Antiretroviral Therapy; CSB: Corn-Soya Blend; CTC: Community-based Therapeutic Care; DHS: Demographic and Health Survey; HAZ: Height-for-Age Z score; HIV: Human Immunodeficiency Virus; LNS (SQ-LNS/ MQ-LNS/ LQ-LNS): Lipid-based Nutrient Supplements- Small Quantity, Medium Quantity or Large Quantity; LP: Linear Programing; MAM: Moderate Acute Malnutrition; NGO: Non-governmental Organization; OTP: Outpatient Therapeutic Program; RUF: Ready-to-Use Foods; RUTF: Ready-to-Use Therapeutic Foods; RUSF: Ready-to- Use Supplementary Foods; SAM: Severe Acute Malnutrition; SNNPR: Southern, Nations, Nationalities and People’s Region; TFC: Therapeutic Feeding Centres; WHO: World Health Organization; WHZ: Weight-for-Height Z score; WTP: Willingness to Pay; 25OHD: 25-hydroxyvitamin D.