1. Introduction
Oxidative stress is the state of imbalance between the reactive oxygen species (ROS) and the ability of a biological system to detoxify readily the reactive intermediates. Development of oxidative stress because of free oxygen radical generation has been implicated in the pathogenesis of many diseases including Parkinson's disease, Alzheimer's disease, atherosclerosis, heart failure, myocardial infarction and even cancer. Free radicals (FRs) are unstable ions that tend to interact with cellular structures.
ROS and FR are continuously formed in the body of mammals by a partial reduction of oxygen. The superoxide radical (•O2ˉ), one of the ROS, is known to be generated in brain. It is involved in the reduction of certain iron complexes including cytochrome C and ferric ethylenediaminetetraacetic acid (Fe3+-EDTA) [1].
The antioxidant system of the body defends the ROS produced in the body. Superoxide dismutases (SOD) help the body to remove the superoxide radicals by converting it to hydrogen peroxide (H2O2). Catalases further catalyze the conversion of hydrogen peroxide to water and oxygen. Glutathione peroxidases are also involved in the removal of H2O2. If the production of FRs increases beyond a certain level in the body of the organism, the defensive enzyme systems fail and the condition leads to oxidative stress.
Oxidative stress in turn may lead to increase in the free calcium ions and iron within the cells in mammals and this rise in intracellular free Ca2+ may result in DNA damage by endonuclease activation [2]. Severe oxidative stress can result in cell damage and even cell death [1].
2. Materials and Methods
Clients (n=12 from population of 500) with primarily digestive issues provided mid stream urine samples on the first visit. Their urine sample was tested for levels of MDA. They were then given appropriate supplements and asked to follow a specific dietary routine and supplements for a period of between 21 to 81 days.
The type and range of supplements given were based on;
- Presenting symptoms
- Other tests. Some of these assessments have been discussed in other papers. Others will be discussed in future papers (i.e. ph of urine, gateway tests for Vitamin C deficiency, calcium status, adrenal stress, bio-impedance assessment and heart rate variability).
Supplements were to be taken on an empty stomach. Subsequently their urine samples were tested for MDA levels again.
The length of period was determined by the following factors:
- When a client started the protocol. A patient may do the test on day 1 and only start the protocol after day 10.We could only measure them at the subsequent visit.
- Some clients visited our clinic from abroad. So their subsequent visits could be a few months apart.
So we have two test points and we can measure the changes demonstrated by these products in between. Typically clients are asked to follow their blood type diet, take digestive enzymes and probiotics as well as take anti-oxidant products.
The testing vial is a one-use vial [65]. The kit comes with a urine cup, the testing vial, a pipette to add urine to the vial, and specific directions. Ideally the test should be done before eating or drinking in the morning using 1st morning's urine. The results of the reading are on a scale from 0 to 5 (with 0 being the absence of MDA and 5 being the highest level of MDA) (Figure 1).
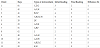
3. Test Results (Table 1)
3.1 Types of Anti-oxidants are listed below
Days: Number of days on regime.
3.1.1 A: Isotonic Solution containing Multivitamins
Beta-Carotene 10,000IU, Vitamin C 150mg, Vitamin D3 1,000IU, Vitamin E 67IU, Vitamin B1 2.6mg, Vitamin B2 3mg, Vitamin B6 4mg, Vitamin B12 75mcg, Niacin 40mg, Folate 216mcg, Biotin 300mcg, Pantothenic Acid 20mg, Calcium 53.8mg, Iodine 150mcg, Magnesium (Carbonate) 25mg, Zinc (Lactate) 7.5mg, Selenium (Amino acid complex) 55mcg, Copper 100mcg, Manganese (Gluconate) 2mg, Chromium (Amino Nicotinate) 120mcg, Potassium (Bicarbonate) 108mg (1 capful in 60ml of water once daily).
3.1.2 B: Isotonic Solution containing Anti-oxidants
Potassium (Bicarbonate) 95mg, Bilberry Extract 25mg, Citrus Bioflavanoid Complex 25mg, Grape Seed Extract 25mg, Pine Bark Extract (Pycogenol) 25mg, Red Wine Extract 25mg (1 capful in 60ml of water once daily).
3.1.3 C: Isotonic Solution containing B-complex
Vitamin B11.95mg, Vit B2 2.25mg, Vit B3 25.5mg, Vit B6 2.1mg, Vit B12 3.6mcg, Folic Acid 600mcg , Biotin 300mcg, Calcium Pantothenate (Vit B5) 20mg, Calcium Sulfate 3.85mg, Magnesium (Oxide) 16mg, Potassium (Bicarbornate) 94mg (1 capful in 60ml of water once daily).
3.1.4 D: Isotonic Solution containing Magnesium
Magnesium (Citrate) 100mg, Magnesium (Glycinate) 100mg, Potassium (Bicarbonate) 75mg (1 capful in 60ml of water once daily).
3.1.5 E: Multivitamin Capsules
Beta-Carotene 5000IU, Vitamin E 60IU, Vitamin C 75mg, Vit D3 400IU, Vit B1 25mg, Vit B2 25mg, Vit B6 50 mg, Vit B12 90mcg, Niacinamide 25mg, Biotin 10mcg, Choline Bitartrate 10mg, Inositol 10mg, Pantothenic Acid 25mg, Calcium 125mg, Magnesium 50mg, Potassium 25mg, Molybdenum 25mcg, Vanadium 25mcg, Lutein (from FloraGlo) 250mcg, Lycopene 20mcg, Red Bioflavanoids 5mg, Hesperidin 5mg, Quercetin 5mg, Rosehips 5mg, Rutin 5mg, Green Tea 5mg, Lecithin 5mg. Cranberry 5mg, Zinc 5mg, Selenium 50mcg, Copper 1mg, Manganese 1.5mg, Chromium 25mcg, Potassium 25mg, Iodine 0.05mg (Dosage 1 capsule twice daily).
3.1.6 F: Efalex
Fish Oil 1280mg of which Omega 3 395mg with DHA 280mg, EPA 70mg),Primrose Oil 426mg (Omega 6 330mg, containing GLA 50mg and AA 22mg) , Vitamin E 20mg, Thyme Oil 1mg (Dosage 5 m l twice daily).
3.1.7 G: Fish Oil
EPA, DHA ,Other Omega Fatty Marine Lipid Concentrate 2g, EPA (Eicosapentaenoic acid) 600mg, DHA (Docosahexaenoic acid) 400mg Acids (1 capsule three times daily).
3.1.8 H: Zinc Solution
Zinc (as Zinc Sulfate) 15mg, Deionized water (5 ml once daily).
3.1.9 I: Liver Formula
Capparis spinosa 130mg, Cichorium intybus 130mg, Mandur bhasma 66mg, Solanum nigrum 64mg, Terminalis arjuna 64mg, Cassia occidentalis 32mg, Achillea millefolium 32mg, Tamarix gallica 32mg (I capsule twice daily).
3.1.10 J: Olive Leaf extract
Olive Extract (leaf) 225mg (oleuropein 13.5mg (6%), Olive Powder (leaf) 225mcg (I capsule thrice daily).
3.1.11 K: Wobenzymes
Pancreatin 300mg , Papain 180mg , Bromelain 135mg, Trypsin 72mg, Chymotrypsin 3mg, Rutoside trihydrate 150mg (1 capsule trice daily).
3.2 Readings
Refers to Colometric reading of MDA levels from 0-5.
The 12 samples were taken from population of 500. Let X be the value of the difference between initial and final readings. Let the mean be μ and the population variance δ2.
δ2 =4.09, so X ~ N(μ, 4.09)
Null hypothesis Ho: μ = 0 (Anti-oxidants make no difference to
urinary MDA levels)
H1: μ > 0 (Anti-oxidants reduce urinary MDA levels)
The sampling distribution of the mean
If Ho i s true μ = 0, X ~ N(0, 4.09/12)
The test statistic is
P(Z > 2.326) =0.01
Since Z > 2.326 we reject Ho and conclude that there is significant evidence at 1% level of confidence that there is a change in the mean value of the MDA colorimetric readings after having the clients ingest anti-oxidants.
4. Discussion
4.1 Lipid peroxidation
Lipid peroxidation is a chain reaction occurring during oxidative stress leading to the formation of various active compounds including propanedial and 4-hyrdoxynonenal (HNE) resulting in the cellular damage. It provides a continuous supply of FRs that lead to further peroxidation. In a toxicity study of the oxidative products, peroxide (LOOH) was found to be more toxic than 4-hydroxynonenal (HNE) and much more than malondialdehyde (MDA) [3]. Lipid peroxidation can be initiated by any chemical species that can extract a hydrogen atom from side chain of a polyunsaturated fatty acid (PUFA) which is generally present in the cell membranes.
Arachidonic acid is a polyunsaturated omega-6 fatty acid present in the cell membranes, brain, muscles and liver that contains many uninterrupted methylene double bonds that serve as a source of hydrogen atoms for the FRs. Arachidonic acid induces the platelets to produce large amounts of MDA. Arachidonate conversion to MDA catalyzed by human platelet microsomes is inhibited by vinblastine [4]. MDA formation by platelets is also prevented by aspirin or indomethacin [5].
Lipid peroxides, which are derived from polyunsaturated fatty acids, are unstable. They readily decompose to form a complex series of compounds, which include MDA. MDA is the major metabolite of arachidonic acid and serves as a reliable biomarker for oxidative stress. MDA is a mutagenic, tumorigenic and highly reactive three-carbon aldehyde produced during polyunsaturated fatty acid peroxidation and arachidonic acid metabolism.
MDA has melting point that ranges between 72-74 0C [6] appears solid and needle like [7]. It has the pKa value of 4.46 and exists as its conjugate base (–O– CH=CH–CHO) [6]. MDA takes part in different biological reactions inside the cells including covalent binding to proteins, RNA and DNA [8].
4.2 Malondialdehyde (MDA) as a bio-indicator
The monitoring of MDA levels in different biological systems can be used as an important indicator of lipid peroxidation both in-vitro and in-vivo for various health disorders. The endogenous formation of MDA during intracellular oxidative stress and its reaction with DNA forms MDA DNA adducts which makes it an important biomarker of endogenous DNA damage [9]. Determination of MDA in blood plasma or tissue homogenates or urine is one of the useful methods to predict the oxidative stress levels.
MDA falls in the category of Thiobarbituric Acid Reactive Substances (TBARS) and the later are an index of lipid peroxidation. Various techniques are used to measure the levels of MDA in different samples including serum, plasma or tissues. Thiobarbituric acid (TBA) assay is the commonly used method for determination of MDA. Hydrogen peroxide (H2O2) and tert-butyl hydroperoxide (t-BOOH) were found to be responsible for increased MDA levels in hepatoma cell (HepG2) culture [10] and higher MDA levels were found in conditions of oxidative stress.
Derivatisation of MDA with 2, 4-dinitrophenylhydrazine (DNPH) has also been described for determination of MDA in human urine [11].
Similarly, HPLC was used for determination of plasma MDA levels in normal male and female volunteers [12].
Another simple and sensitive method for determining MDA concentration is gas chromatography mass spectrometry (GC-MS) for the determination of MDA in blood [13]. Researchers used headspace-solid phase microextraction gas chromatography mass spectrometry (HS-SPME-GC-MS) with Acetone-d [6] as internal standard. The psychostimulant drug methamphetamine (MA) can harm brain dopamine neurons, possibly by causing oxidative damage [14].
Significantly, higher levels of 4-hydroxynonenal (67%) and MDA (75%) in the dopamine rich caudate nucleus and frontal cortex (48 and 36% respectively) were found using GC-MS.
MDA was found to form Schiff-base adducts with lysine residues and cross-link proteins in-vitro. Reduced forms of Lysine-MDA [3-(N epsilon-lysine) propan-1-ol (LM)] and Lysine-MDA-Lysine aminopropane cross- link [1,3-di(N epsilon-lysine) propane (LML)] were prepared and GC/MS assay was used to quantify the reduced compounds in protein [15].
LM and LML increased with the formation of conjugated dienes during the copper catalyzed oxidation of LDL.
4.3 MDA in different disease patterns
Higher levels of MDA concentration has been reported in different studies including lung cancer patients [16]. High levels of MDA were also found to play a role in atherogenesis in rabbit aorta [17]. A positive correlation between atheromatous lesions and MDA concentration was found. Incubation of cultured endothelial cells of human umbilical vein with MDA (200μM) for 24 hours led to an increase in the cell stiffness [18].
A marked increase in MDA levels was observed following the course of circulatory shock in human muscular tissue [19]. Plasma extracellular superoxide dismutase (SOD3) activity, MDA and CD4 counts were studied were recorded in HIV positive subjects in Kano state, Nigeria and a negative correlations between the serum MDA concentration and CD4 cell count were found [20].
Exposure of human erythrocyte solutions to Trichlorfon (an insecticide) was found to increase the levels of MDA in a dose dependent manner, decrease glutathione peroxidase (GSH-Px) and reduce glutathione (GSH) levels [21,64].
4.3.1 MDA and ocular pathologies
Lipofuscin (LF) accumulation is associated with various retinal diseases and LF accumulation in the retinal pigment epithelium (RPE) may interfere with the normal retinal function. Proteins in the LF granules are oxidatively modified by MDA [31,32,64].
4.3.2 MDA in ischemic conditions
Human growth hormone was found to increase lipid peroxidation in rats which in turn increases MDA level of hypoxic-ischemic newborn rat brain tissue [33].
The study revealed that oxygen deprivation induces the production of a low, but detectable amount of MDA in both heart and brain tissues of rats, whereas reperfusion causes a marked increase of MDA in both the tissues [34].
In humans, plasma MDA was affected only in patients suffering from acute myocardial infarction with successful thrombolysis, thus indicating the occurrence of oxygen radical-mediated tissue injury.
Oxidative stress was measured in human and rat hearts which were temporarily made ischemic by measuring oxidized glutathione (GSSG) and MDA levels. It was found that after ischemia, human and rat heart show signs of oxidative stress by releasing GSSG whereas human heart was found to have no MDA under normoxic and ischemic conditions. Cardioplegia induced a 41% decrease in rat heart MDA content [35,64].
4.3.3 MDA in reproductive pathologies
MDA in seminal plasma of normal and abnormal men was measured by thiobarbituric acid test and it was found that abnormal semen samples had significantly lower number of viable spermatozoa as compared to normal semen samples [36]. Similarly, Gomez et al. [37] found highly significant correlations (P<0.001) between the loss of motility of spermatozoa and oxidative stress created either with xanthine oxidase or by prolonged aerobic incubation [64].
4.3.4 MDA and hypertension
Higher serum MDA levels were found in hypertensive patients as compared to normotensive control individuals [38]. Higher levels of serum-MDA and decreased catalase activity were found in hypertensive pregnant women as compared to controls [39]. El- Banaet et al. [40] studied the maternal and cord plasma concentration of MDA in pre-clamptic and healthy pregnant women. The concentration of MDA in pre-clamptics was found to be significantly lower in cord plasma as compared to maternal plasma.
4.3.5 MDA, prostaglandins and lipid hydroperoxides
MDA and 15(S)-8-iso-prostaglandin (2aplha) belong to the group of post frequently analysed biomarkers of oxidative stress in basic and applied clinical research.
The effects of haemolysis on free MDA and total (free + esterified) 15(S)-8-iso-Prostaglandin (2aplha) concentration in human plasma were examined and was found that in both in-vivo (r = 0.74) and invitro (r = 0.87) conditions, there was a positive significant correlation between haemolysis degree (0 - 0.2%) and plasma-MDA concentration (50 - 250 nmol/L).
It was hypothesized that free haemoglobin catalyzes the formation of MDA and 15(S)-8-iso-PGF (2aplha) from free and esterified arachidonic acid [41].
Tesoriere et al. [42] examined the exposure of RBC to MDA in glucose containing phosphate saline buffer and found a 16% hemolysis within 6 hours. Melatonin was found to prevent the formation of CD lipid hydro-peroxides and protect the RBCs from MDA induced time-dependent haemolysis [43].
The influence of Leukotriene C4 (LTC4) on aggregation and MDA formation in human blood platelets was studied by Ponicke and Forster [44] and it was found that MDA formation was enhanced by LTC4.
The presence of certain protozoans including Leishmania is associated with higher levels of MDA. Leishmania sp. are obligate intercellular protozoans that infect and replicate within mammalian macrophages. Macrophages, neutrophils and other phagocytic cells are capable of generating large amount of reactive oxygen species (ROS) and RNS (Reactive nitrogen species).
The overproduction of ROS and RNS results in oxidative stress and the acceleration of lipid peroxidation in cutaneous leishmaniasis patients, resulting from altered enzymatic antioxidant activities as compared to controls [45,64].
4.4 MDA-lipid associations
The MDA association with plasma lipoproteins alters the lipid structures via apoprotein or apoprotein/ lipid associations [46].
Malondialdehyde- modified low-density lipoproteins (MDALDL) were also detected in sera of 40 healthy individuals by an immunosorbent assay technique in which a monoclonal antibody was used against MDA-LDL complex [47].
Adducts of MDA with apolipoprotein (apo-B) were also found in plasma of nine patients with severe atherosclerosis [48].
Malondialdehyde modified high density lipoproteins (MDAHDL) were studied and MDA-HDL was found to be less effective in decreasing cellular cholesterol content [49].
It also contributed to the progress of atherogenesis by decreasing cholesterol efflux from peripheral tissues.
In a study, the levels of lipid profile and antioxidant status in the serum of 15 hyperlipidemic patients and 30 matched normolipidemic control subjects was measured and a positive correlation was found between MDA and Atherogenic Index (AI) [50].
4.5 MDA-DNA interactions
It has been reported that MDA reacts with DNA bases to form a series of adducts of deoxyadenosine (M1A), deoxycytidine (M1C) and deoxyguanosine (M1dG) [51].
3-(2-deoxy-beta-d-erythropentofuranosyl pyrimido) [1, 2- alpha] purin-10 (3H) one, M1dG is the major adduct derived from the reaction of DNA with MDA and the DNA peroxidation product base propenal.
Cyclooxygenase- 2 (COX-2) activity in human colon cells also results in formation of MDA and generation of M1dG adducts [52]. Maddukuri et al. [53] conducted an experiment to evaluate bypass of M1dG by human Y-family DNA polymerases kappa, iota and Rev 1. The result indicated that DNA hPol kappa or the combined action of hPoliota or Rev 1 and hPol kappa bypass M1dG residues in DNA and generate products that are consistent with some of mutations induced by M1dG in mammalian cells.
Sun et al. [54] used an immuno-enriched 32-Ppostlabeling HPLC method for detection of M1dG in human breast and liver tissue.
The lipid peroxidation products can accumulate at high levels in the breast tissues of women with breast cancer. It was found that breast tissues from 51 cancer patients exhibited significantly higher levels of the putative MDA adducts than those found in noncancerous controls [55].
The levels of malondialdehyde deoxyguanosine adduct isolated from the DNA blood sample of 10 healthy human donors demonstrated significant differences between the levels in males and females [56].
MDA increased the mutation frequency (up to 15-fold) in supF reporter gene as compared to untreated DNA [57].
Daily smokers had a significantly higher concentration of P-MDA than non-smokers did [58] due to increased lipid peroxidation. An immune histochemical method was used for detection of adducts in human oral mucosa cells with respect to smoking habit. The level of DNA damage was found to be higher among 25 smokers [9].
In men, positive associations of M1dG adduct level with height and age, and an inverse association with body mass index was found. Legumes, fruits, salads and whole meal bread were inversely associated with M1dG adducts, whereas consumption of offal, white meat, beer and alcohol were positively associated with elevated levels. In women, there was an inverse association of the adducts with the ratio of polyunsaturated: saturated fatty acids (and a weak positive correlation with saturated fat [59].
Deoxyguanosine-MDA adducts (dG-MDA) was identified in both rat and human urine samples by HPLC and fluorescence detection [60]. Researchers [61] isolated a 1:1 guanine malondialdehyde adduct from rat and human urine and suggested its endogenous origin as indicated by its presence in rat urine fed on MDA free diet [61,64].
4.5.1 MDA-DNA adducts and cancer
Xing et al. [62] found that M1dG adducts may be involved in the initiation and progression of cancer with its mutagenic and carcinogenic effects in human esophageal tissue.
In adenoma patients, the M1dG [63] adduct levels were compared with controls and a trend for higher values was found in individuals with adenomas.
4.6 Summary of discussion
From the discussion above, although for the purposes of this clinical study we have focussed on digestive related issues, many of the clients often have other related symptoms that are also affected by changes in MDA. Apart from primarily digestive issues they also had allergies, some had lipid related issues and others from a variety of other health concerns. All of which could have a relationship with MDA levels.
We have added a few variations from the suggested use of test. Typically, it has been suggested that the testing be done when the subject has abstained from any nutritional supplements.
However we typically test new clients on the first visit without asking them to change anything. We want a snapshot of whether their current lifestyle diet and nutritional regime status supported their antioxidant status. When we re-do the test, we have the client continue to take the new supplements up to the day before the test.
It appears that urinary MDA levels are confounded by dietary MDA content [66,67], We were also providing support to the digestive system as well. This was in the form of digestive enzymes and probiotics as well as dietary modulation. So this may also result in the reduction of free radicals. We may also be supporting the stress hormone system. This may also reduce oxidative stress.
Dietary intake of certain antioxidants such as vitamins was associated with reduced levels of markers of DNA oxidation (M1dG and 8-oxodG) measured in peripheral white blood cells of healthy subjects, which could contribute to the protective role of vitamins on cancer risk [68].
So the discussion was to demonstrate that MDA and its adducts can be implicated in many diverse disease conditions. So assessing MDA levels before and after treatment protocols is a reasonable way of assessing the efficacy of a protocol in lowering MDA levels and by implication improving therapeutic outcomes.
5. Conclusion
Preliminary conclusion suggests that Malondialdehyde (MDA) is a useful biomarker for lipid peroxidation and oxidative stress. Increased levels of oxidative stress have been associated with various disease patterns [64]. Oxidative stress in the body can also be reduced with appropriate anti-oxidants. However future research has to determine to what extent dietary and nutrient modulations to the digestive system and the stress hormonal systems can impact the oxidative stress status. Longer term studies need to be conducted to assess the impact of anti-oxidants on oxidative stress.
6. Ethical Considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the author. The clients involved in the study signed an informed consent form that they wished to be treated for a variety of health issues at Sundardas Naturopathic Clinic. All the products and tests used are already approved for use. This was not a clinical trial. As such there was no necessity to register it.
Competing Interests
The author declare that there is no competing interests regarding the publication of this article.