1. Introduction
Nowadays consumers are becoming more interested in incorporating healthy food components such as probiotics in their diet. To provide positive effects, probiotics must remain viable in the food products at a level of at least 106 cfu g-1 until the time of consumption [1] . Numerous strains of probiotic bacteria have been successfully added into different types of cheeses [2-7]. However, their viability until the time of consumption may be adversely affected by processing conditions as well as by the product environment and storage. Many compositional and process factors significantly affect the viability of probiotics in cheese including the kind and the amount of probiotic inoculation, microbiota competition, pH, incubation and storage temperature, salt, packaging materials and other [8] . Therefore, the viability of the probiotics is a complex phenomenon, highly affected by both the kind of strain and type of cheese and it is important to be studied on a case-by-case basis. [9] . Strains of the genus Lactobacillus and Bifidobacterium are widely used as probiotic microorganisms in probiotic foods. L. acidophilus NCFM, L. acidophillus 145, Lactobacillus paracasei subsp. paracasei LbC 81 and Lactobacillus rhamnosus LC 705 are some of the probiotic lactobacilli stains with proven therapeutic properties [10,11,12].
Galotyri is one of the oldest traditional cheeses in Greece, made from ewe’s or goat’s milk or mixture of both. It is an acid curd cheese with moisture content < 75% and fat content in dry matter >40%. It has spreadable texture, characterized by a sourish and pleasant refreshing taste and aroma [13] . Galotyri was produced in many regions of Greece in many ways. The variations in manufacturing methods resulted in different types of Galotyri cheese [14] . In 1996, Galotyri cheese was recognized as protected designation of origin (PDO) [15] . According to EU regulation, it should be produced exclusively in the regions of Epirus and Thessaly.
Since Galotyri cheese has pleasant organoleptic characteristics, it is much appreciated by the Greek consumers and there is a great demand for its production. As a result, many dairies in different regions of the country produce cheeses that resemble Galotyri, but using processes different from the PDO cheese. Selective commercial starter cultures and different production procedures for Galotyri cheese making have been suggested [16,17] but up to date, there is no data about the effect of the addition of probiotic lactic acid bacteria to the characteristics of the product.
The aim of this study was to make Galotyri cheese using a commercial mesophilic starter culture along with four probiotic strains of Lactobacillus acidophilus, Lactobacillus paracasei subsp. paracasei or Lactobacillus rhamnosus to assess the viability of each of the selected probiotics into the cheeses and to evaluate their effects on the technological, physicochemical, and sensory characteristics of the cheeses during 30 days of refrigerated storage.
2. Materials and Methods
2.1 Starter cultures
The mesophilic culture BT002 (Rhodia Food, Lyon, France) (E) consisting of a mixture Lactococcus lactis spp. lactis/Lactococcus lactis spp. cremoris (60%) and Lactococcus lactis spp. lactis biovar diacetylactis (40%) was used as starter for cheesemaking. The probiotic strains Lactobacillus acidophilus NCFM (Rhodia Food, Lyon, France) (A), Lactobacillus acidophilus 145 (Danisco, Dangé, France) (B) Lactobacillus paracasei subsp. paracasei LbC 81 (Rhodia Food, Lyon, France) (C), or Lactobacillus rhamnosus LC 705 (Danisco, Dangé, France) (D) were also added to cheese milk to achieve a concentration of 105 to 106 cfu mL-1. All cultures were added in freeze-dried form except Lactobacillus acidophilus 145 that was added in deep frozen pellets.
2.2 Cheese manufacture
The ewes’ milk used to produce Galotyri cheese was obtained from a dairy farm near Athens. Five types of Galotyri cheese were manufactured in seven repetitions as follows: cheese E (containing only the mesophilic starter culture BT002) and cheeses A, B, C and D containing the mesophilic starter culture BT002 along with the probiotic strains L. acidophilus NCFM, L. acidophilus 145, L. paracasei subsp. paracasei LbC 81 or L. rhamnosus LC 705 respectively. After fat standardization the milk was poured in a double-wall stainlesssteel vessel and heated to 90ºC for 10 min. Milk portions of 10 kg were transferred to five cleaned sterilized vats, cooled to 30oC and inoculated with the mesophilic starter culture. Five minutes later the probiotic strains were added to the cheese milk. Then calf rennet powder of 90% chymosin and 10% pepsin with clotting activity 1:100,000.00 (Vlachopoula, Athens, Greece) was dissolved in cold water and added at a quantity of 0.006 g kg-1 milk. Coagulation at 30oC was stopped after 24 hours, when the pH fell to a value of 4.4. Then the curds were transferred in clean cloth bags using a ladle and were hung from a rafter in the store room for curd draining at 30 ºC for 24 hours. They were taken out from the bags on a clean board, mixed well with dry salt 1% (1.0 g kg-1), weighted and packed in plastic sterilized containers. The cheeses were transferred to a cold room (4oC) for storage up to 30 days. All the analysis was monitored until 30days of cold storage.
2.3 Microbiological analysis
The microbiological analysis was performed immediately after the addition of probiotic bacteria and at 1, 8, 15 and 30 days of storage at 4ºC. All the counts were expressed as colony forming units per mL or gram of cheese (cfu mL-1 or g-1). The probiotic bacteria were enumerated on MRS agar (Biokar Diagnostics, Beauvais, France), that supports optimum growth for these species [18] , after incubation at 37oC for 72h and were identified by the specific morphology of their colonies. The colonies of L. acidophilus strains were large, light brown, rough with irregular shape, L. paracasei LbC 81 and L. rhamnosus LC 705 were identified as inflatable, round, creamy, white colonies and all of them were very distinguishable from the smaller, flat, round, gray colonies of the mesophilic lactic acid bacteria that were grown as well. Further confirmation of the lactobacilli was carried out by optical microscopy of Gram-stained cultures and API identification test (API 50 CHL, API System, BioMérieux, France).
Mesophilic cocci were enumerated on M17 agar (Biokar Diagnostics, Beauvais, France) after incubation at 30oC for 3days, yeasts on YGC-agar (Merck, Darmstadt, Germany) after incubation at 25oC for 5-7days and coliforms on Violet Red Bile Agar (Oxoid, Hampshire, England) after incubation at 37oC for 1day according to standard procedures IDF 149Α: 1991, IDF 94Β: 1991 and IDF 73Α: 1985 respectively [19,20,21].
2.4 Physicochemical analysis
Cheese milk was analyzed for fat, protein and lactose using Milkoscan apparatus (Milkoscan 133, Foss Electric, Hillerod, Denmark). The pH value was monitored during coagulation using a Hanna model HI 98240 pH-meter (Hanna Instruments, Woonsocket, USA). The loss of the cheese whey (volume in mL) was measured every one hour during the draining of the curd.
Cheeses were analyzed after draining and before salting (1st day cheeses) for fat by Gerber van Gulik’s method [22] , moisture [23] protein [24] and ash content [25] . Lactose content was determined by subtracting the sum of total percent of fat, protein and ash contents from that of total solids content. The yield of each cheese was determined after curd draining and calculated as the weight of cheese obtained from the cheese milk used for production and expressed in percentage. The pH of cheeses was measured using a Hanna model HI 98240 pH meter (Hanna Instruments, USA) during the processing and storage. All analyses were performed in triplicate.
2.5 Analysis of volatile compounds
The cheeses were subjected to analysis of volatile compounds at 1, 8, 15 and 30days of storage at 4ºC using GC/MS (HSGC-MS, QP 5050, Shimadzu Scientific Instruments, Inc., Columbia, MD, USA) [26] .
2.6 Sensory analysis
Cheese samples were subjected to sensory analysis at 1, 8 and 30 days of storage by a panel group of the Dairy Laboratory of the Agricultural University of Athens, previous trained on Galotyri cheese attributes and defects. The samples were presented in a random order to panelists and were evaluated for taste, texture and color, according to the IDF standard 99C, 1997. A10-point grating scale was used as follows: 1-2 bad, 3-4 not satisfying, 5-6 good, 7-8 very good and 9-10 excellent. A section for the panelist’s comments was also present in the evaluation sheet. ”
2.7 Statistical analysis
Data were subjected to analysis of variance (ANOVA) with two factors (type of strain and storage time). Statistically significant differences between means were determined using LSD test (P<0.05). The relationship between characteristics of the cheeses was estimated by regression analysis. The software Statgraphics Plus for Windows v.5.2 1995 (Manugistics, Inc., Rockville, MD, USA) was used.
3. Results and Discussion
3.1 Temperature selection
The selection of the appropriate temperature during cheese making was a critical point for this endeavor. Since the average optimum temperature for the selected strains varied from 25 – 37oC we tested three different temperature levels 25, 30 and 37oC for cheese making. The final products were tested organoleptically after their preparation. We noted that in trials with temperature 25ºC the counts of probiotic bacteria decreased. L. acidophilus strains were the most sensitive, needing at least 30ºC temperature to survive. At higher temperature (37ºC), the growing up of all probiotic strains was good but the cheeses were dry and of low quality. Therefore, we selected temperature at 30ºC for cheese making so as to achieve products rich in probiotics that exhibit acceptable sensory characteristics.
3.2 Microbiological characteristics
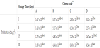
The viability of probiotic bacteria in the cheeses during 30 days of storage at 4oC is shown in table 1. The mean initial inocula were 2.5 x 105 cfu mL-1 for L. acidophilus NCFM, 7.0 x 105 cfu mL-1 for L. acidophilus 145, 2.6 x 105 cfu mL-1 for L. paracasei LbC 81 and 1.3 x 106 cfu mL-1 for L. rhamnosus LC 705. After draining of the curds all Lactobacilli counts were >106 cfu g-1 although considerable number of bacterial cells were lost in the whey. The counts of L. acidophilus strains in cheeses A and B were 1 (and below) log cycle higher than the initial inocula. L. rhamnosus LC 705 numbers in cheese D were almost 1.5 log cycle higher, while L. paracasei LbC 81, had a better adaptation in cheese C and increased about 2 log cycles, scaling the mean value 2.80x107 cfu g-1 (Table 1). The high bacteria counts were maintained without significant changes throughout the storage period. Cheese environment did not affect the growth of lactobacilli and they remained viable above the minimum recommended level of 106 cfu g-1 satisfying the criteria established for probiotic foods. After 30days of storage L. acidophilus NCFM populations were approximately the same, L. acidophilus 145 declined about 0.4 log cycle while in contrast L. paracasei LbC 81 increased about 0.5 log cycle. Previous studies reported similar findings for different probiotic strains of L. acidophilus and L. paracasei during the refrigerated storage in various types of cheeses [27-29,5,4]. After 30days of storage, L. rhamnosus LC 705 declined greater than 0.5 log cycle, but even in this case the population was higher than 106 cfu/g.
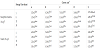
The viability of mesophilic starter culture in the cheeses during storage at 4oC is shown in table 2. There was no significant difference in starters’ counts among 1-day-old cheeses. At day 1, these counts were higher than 108 cfu g-1, decreased up to 1.5 log order after 8days and then reduced insignificantly until the end of storage. The decrease of the numbers of mesophilic starters could be related to the low pH, as mesophilic cocci are not acid tolerant and are inhibited when the pH falls below 5.5 [30] .
The above results indicated that selected probiotics did not exert any effect on the growth of starters and the conditions of the cheese making were suitable for them. Yeasts remained in low levels during storage counted from 103 cfu g-1 to 104 cfu g-1 during storage. Coliforms were not detected in any case (data not shown).
3.3 Physicochemical characteristics
The mean chemical composition of the cheese milk was fat 6.0%, protein 5.52%, lactose 4.96% and pH value was 6.55. The acidification rate of milk was similar in all the curds. The pH decreased slowly during the first 4 h, after 8 hours was below 5.30 and towards the end of coagulation (after 24 hours) dropped at the desired levels about 4.40 in all the curds. Mean pH value was 4.29 after draining and before salting and after salting 4.47. During storage pH remained relatively constant with no significant differences among the different type of cheeses ranging from 4.40 to 4.50 until the end of storage. The rate of whey loss during draining (Figure 1) was similar in all curds. Approximately 68% of the whey drained over 24h was drained within the first 8h, fact important for commercial scale production, where it is desirable to reduce the process time of the products.
The physicochemical characteristics of the cheeses are shown in table 3. The addition of probiotic lactobacilli in cheese indicated no significant change in the composition. The moisture of cheeses ranged from 71.85% to 73.14% as suggested by the Greek Legislation for Galotyri cheeses [31] . The yield was high and ranged from 47.40% to 49.11% demonstrating the economic importance of this type of cheeses.
3.4 Volatile compounds
A total of 32 volatile compounds were detected in the cheeses, including esters, carboxylic acids, ketones, aldehydes, alcohols, hydrocarbons and terpenes. Ethanol, acetaldehyde, acetone, acetoin, diacetyl, acetic acid, 3-methyl butanol, 3-methyl butanal have been tentatively identified as the aroma-active compounds of different Galotyri cheeses [32] . Their levels are presented on table 4. Some of them as acetaldehyde, diacetyl and acetoin were found in higher levels in cheeses containing probiotic strains. Significantly higher levels of acetaldehyde were found in cheeses A and B with L. acidophilus (P<0.05). Acetaldehyde which imparts green apple, nutty and roasted flavor to foods is an important volatile compound for the flavor formation of dairy products such as yogurt, fermented milk and acidcurd fresh cheeses [32] . High levels of acetaldehyde were also found by Marshall & Cole [33] in fermented dairy products containing L. acidophilus strains. Acetaldehyde was decreased in all experimental cheeses during storage period.
Diacetyl and its reduction product acetoin, are commonly produced from citrate metabolism by Cit (+) bacteria, although it has been suggested that they may also derive from aspartic acid by transamination by mesophilic lactobacilli [34,35]. Galotyri cheeses containing probiotic lactobacilli were characterized by higher levels of acetoin and diacetyl (P<0.05) compared to control cheese E. Relative abundance of diacetyl was detected in cheeses C and D. According to Burns et al. [36] , Milesi et al. [37,38] and Peralta et al. [39] high levels of acetoin and diacetyl were detected in cheeses inoculated with L. paracasei and/or L rhamnosus. However, variability in the production of the volatiles compounds is noted which can be attributed to the type of strain used, the physicochemical factors and the fermentation or storage conditions. After 15days of storage acetoin was decreased in all cheeses.
Acetate is considered to enhance cheese flavor, although high concentrations may cause off-flavors. Most of the cheeses containing probiotic lactobacilli have high acetic acid levels due to their heterofermentative pathway [2] . Ong et al. [40] , Milesi et al. [38] and Gomes et al. [41] found that concentrations of acetic acid were high in cheeses supplemented with L. casei, L. acidophilus or L. paracasei. In the present study, the level of acetic acid was higher in cheese D with L. rhamnosus. At the end of the storage period, acetic acid was gradually decreased in cheeses A, B and C.
3-methyl-1-butanol, responsible for the pleasant aroma, giving alcoholic floral note to some soft cheeses [42] was detected in relative small amounts. After 15days of storage 3-methyl-1-butanol was increased in probiotic cheeses.
3.5 Sensory evaluation
The sensory evaluation of 1- 8- and 30-day-old cheeses is presented on table 5. All Galotyri cheeses received high total sensory scores up to 30days of refrigerated storage. Whey separation was not observed, all the cheeses remained well spreadable and characterized as ‘very good’.
Scores of sensory evaluation were reduced insignificantly during storage. At the end of storage, cheese C containing L. paracasei subsp. paracasei LbC 81 gained higher scores among the cheeses studied.
The panelists commended that they preferred more viscous and granulated products as well as refreshing and mildly acidic. Thus, there was a positive correlation between the scores of taste and texture and the measurements of pH (r=0.57, P<0.05 and r=0.61, P<0.05 respectively) and a negative correlation between taste and texture and the moisture content (r=-0.65, P<0.05, r=-0.76, P<0.001). In conclusion the tested probiotic strains did not significantly change the sensory properties of Galotyri cheese. Several studies showed no significant effects [43,5], while other studies reported improved cheese flavour [44] or some flavour defects [40,41,45].
11. Conclusions
The results of this study showed that the probiotic strains Lactobacillus acidophilus NCFM, Lactobacillus acidophilus 145, Lactobacillus paracasei subsp. paracasei LbC 81 or Lactobacillus rhamnosus LC 705 added into Galotyri cheese did not alter the chemical composition of cheese and had no adverse effect on sensory features during all storage. Galotyri cheeses with probiotics contained acetoin, diacetyl and acetaldehyde in higher levels compared to cheese containing starters only. The probiotic bacteria survived adequately above the recommended level for the therapeutic minimum throughout refrigerated storage. Therefore, this cheese type is an efficacious food matrix for maintaining the viability of probiotics during storage at 4oC.