1. Introduction
Myocardial single photon emission computed tomography (SPECT) is a well-established diagnostic modality for the evaluation of myocardial ischemia or metabolic disorder in various cardiac diseases as well as for the risk stratification of coronary artery disease (CAD). Specifically, the clinical usefulness of myocardial perfusion imaging, such as stress myocardial perfusion SPECT, for detecting CAD is supported by abundant evidence in literature [1-3]. Recently, the number of non-invasive cardiac imaging tests has steadily increased [4]. This has driven the dissemination of state-of-the-art devices of computed tomography (CT) and nuclear medicine worldwide, as well as facilitated cardiac SPECT/CT hybrid imaging.
However, the diagnostic accuracy of conventional myocardial SPECT without CT has remained suboptimal due to tissue attenuation artifacts caused by the breasts, lateral chest walls, abdomen, and diaphragm [5,6]. For myocardial perfusion SPECT, both American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging recommend the incorporation of attenuation correction (AC) to improve diagnostic accuracy [7]. Although stand-alone SPECT scanners require transmission scans by external radionuclide systems to obtain tissue density maps for AC, SPECT/CT hybrid systems can obtain the maps from CT images. The advantages of the computed tomography attenuation correction (CTAC) method include higher quality attenuation maps due to higher photon flux, lower noise, and improved resolution [8]. In addition, CTAC can improve the diagnostic accuracy of myocardial SPECT [9,10].
As for cardiac SPECT/CT hybrid imaging, either SPECT/CT hybrid systems or SPECT/CT image fusion software can be used to create SPECT/CT hybrid images. This imaging has achieved increasing clinical acceptance because it shows the relationship between anatomical information, including the presence of coronary artery lesions, and functional information, such as myocardial perfusion or metabolism [11-14]. The comprehensive findings derived from this imaging modality can provide guidance for appropriate medical or interventional therapies [15].
The present review provides an overview of the most important clinical aspects of cardiac SPECT/CT imaging, specifically on CTAC and cardiac SPECT/CT hybrid imaging. Provided is an outline of relevant literature that discusses upon both issues, including the hardware and software requirements for cardiac SPECT/CT imaging. As research is ongoing and the technique is continuously expanding into other applications, the conceivable clinical applications of cardiac SPECT/CT hybrid imaging will be discovered.
2. CTAC for Myocardial SPECT
2.1 Attenuation artifacts in myocardial SPECT
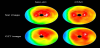
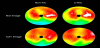
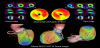
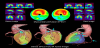
Myocardial SPECT is a widely used and well-established method for the evaluation of myocardial ischemia or metabolic disorder in various cardiac diseases [1-4]. However, the diagnostic accuracy of conventional myocardial SPECT has remained suboptimal due to various artifacts including attenuation artifacts. Non-homogeneous photon attenuation in the thorax is one of the major drawbacks of myocardial SPECT, limiting the diagnostic accuracy, interpretive confidence, and quantification reliability. Consequently, attenuation artifacts may reduce the specificity of myocardial SPECT since regional reduced accumulation due to attenuation artifacts may be misinterpreted as a perfusion or metabolism defect [16]. Figure 1 shows a representative case with an attenuation artifact in the inferoposterior wall. Meanwhile, attenuation artifacts may also reduce the sensitivity of myocardial SPECT because true accumulation defects may be concealed by them [17]. In Figure 2, an attenuation artifact on the myocardial infarction makes diagnosis complicated.
2.2 Correction of attenuation artifacts
Conventionally, attenuation artifacts in myocardial SPECT can be corrected using supine-prone acquisition [18] or attenuation maps obtained using external radionuclide sources [19]. In the past few decades, however, attenuation maps, derived from unenhanced CT scans, have been introduced as a reliable method for AC of myocardial SPECT for clinical practice [20-22]. Figure 1 shows correction of diaphragmatic attenuation artifacts using CT attenuation maps in myocardial perfusion SPECT images. CTAC is instrumental in distinguishing attenuation artifacts from true myocardial perfusion defects in the inferoposterior wall. However, apical perfusion defect, probably due to anatomical apical thinness or wall motion, is often observed in CTAC images and it should be carefully interpreted. CTAC has been proven to have higher diagnostic accuracy than supine-prone acquisition in myocardial SPECT; these modalities have definitive diagnostic rates of 90% and 82%, respectively, for significant CAD (>50% coronary artery stenosis in coronary angiograms) [10]. Compared to AC with external radionuclide sources, CTAC has several advantages, such as higher photon flux, no decay of transmission source, shorter scan times, lower noise, and improved resolution [23,24]. CTAC can be conducted using a SPECT/CT hybrid system or stand-alone SPECT and CT scanners. Using SPECT/CT hybrid systems increases the specificity of myocardial SPECT to 80–90% [9]. SPECT/CT studies have shown that low-dose CT acquisitions are also feasible for CTAC [25]. The main limitation of CTAC is misregistration between myocardial SPECT and CT attenuation maps due to respiratory and cardiac motion [24,26]. Registration correction using software may be required in cases with 1.3 ± 0.4 pixels of misalignment to avoid false accumulation defects [27]. Another limitation of CTAC is potential misalignment between emission and transmission data due to the difference of spatial resolutions, causing the risk of incomplete correction and artificial perfusion defects. Thus, quality control can be used to avoid reconstruction artifacts. PET/CT [28,29] and SPECT/CT [30,31] studies have shown that the frequency of misalignment can be high. Recently, it has been shown that the effects of misalignment are less severe for SPECT/CT than for PET/CT, mainly because of the low spatial resolution of SPECT [32]. The alignment of emission and transmission data is usually performed manually with a high success rate. Furthermore, automated methods for registration are being developed [33,34].
2.3 CTAC for myocardial SPECT using solid-state gamma cameras
Recently, new gamma cameras, optimized for cardiac imaging and equipped with solid-state detectors, have been introduced for clinical use. Discovery NM 530c or 630 (GE healthcare) and D-SPECT (Spectrum Dynamics), equipped with cadmium-zinc-telluride (CZT) detecting elements, are commercially available [35-38] and a prototype gamma camera: R1-M (Hitachi, Ltd.), equipped with cadmium-telluride detecting elements, is currently being developed [39,40]. Compared with conventional NaI gamma cameras, the CZT gamma cameras have multiple advantages, such as improved spatial resolution, high energy resolution, and high sensitivity which reduces patients’ discomfort and minimizes motion artifacts [37,40,41]. However, since the acquisition using the CZT cameras is performed by positioning gamma-ray detectors on the anterior and left lateral side of the thorax, the myocardial SPECT images show noticeable attenuation artifacts in the septal, inferoposterior, and posterolateral wall of the left ventricular myocardium [42]. The degree of attenuation artifacts in CZT images is markedly higher than in conventional NaI images (Figure 1). AC using supine-prone acquisition or CTAC is a possible solution [43,44]. Although the majority of dedicated CZT gamma cameras do not include an integrated CT scanner, CTAC can be conducted using an external-CT-derived attenuation map and CTAC software. Using CTAC, the diagnostic accuracy of CZT myocardial SPECT can be increased (Figure 1 and 2) [44].
3. Cardiac SPECT/CT hybrid imaging
3.1 Clinical background
Development in non-invasive modalities and imaging devices has driven the dissemination of coronary CT angiography (CCTA) and myocardial SPECT for patients with CAD. CCTA is an acceptable alternative to the invasive coronary angiography for the visualization of coronary morphology [45]. However, CCTA solely addresses the morphological findings of coronary arteries and fails to assess the myocardial ischemia caused by coronary artery stenoses [46]. A number of studies have consistently demonstrated that the severity of coronary stenosis is a poor predictor of myocardial ischemia. The same degrees of coronary-artery luminal stenoses may have differing hemodynamic effects among individuals, especially in the moderately stenotic lesions, and collateral vessel formation can alleviate myocardial ischemia [45,47]. Similarly, a comparative study of CCTA and myocardial perfusion SPECT has shown that only 32% of significant coronary stenoses are associated with myocardial ischemia [12]. Furthermore, recent studies using fractional flow reserve have shown that stenoses of 50 to 90% do not necessarily cause myocardial ischemia [48,49]. The prospective nuclear substudy of the COURAGE trial showed that percutaneous coronary intervention (PCI) is effective in patients with myocardial ischemia and that the reduction in myocardial ischemic volume can improve the patients’ prognosis. These data indicate that assessing both morphological and functional findings in CAD is crucial in order to offer the optimal treatments to stable CAD patients. Furthermore, cardiac SPECT/CT hybrid imaging can show the direct relationships between the distribution of coronary artery stenoses and myocardial ischemia, improving the diagnostic accuracy.
3.2 Role of myocardial SPECT in cardiac hybrid imaging
In the past few decades, myocardial perfusion SPECT has been established as a reliable non-invasive modality for diagnosing myocardial ischemia, which has sensitivity and specificity of 87–89% and 73–75%, respectively, for significant CAD (>50% coronary artery stenosis in coronary angiograms) [50]. Furthermore, myocardial perfusion SPECT has an independent prognostic value in various clinical settings, such as stable CAD, risk assessment prior to noncardiac surgery, and acute coronary syndrome (ACS) [51]. Notably, normal myocardial perfusion in patients with stable CAD indicates an excellent, mid-term prognosis; the incidence rate of death or non-fatal myocardial infarction is less than 1% in a year [1]. However, myocardial SPECT has several limitations. In patients with multivessel CAD or balanced myocardial ischemia, myocardial SPECT may underestimate the severity of myocardial ischemia although the myocardial segment with the severest ischemia is detectable [52-54]. Furthermore, myocardial SPECT cannot detect non-obstructive coronary atherosclerotic lesions, which do not cause myocardial ischemia, but such lesions may cause ACS or develop into obstructive atherosclerotic lesions [48,55,56]. In addition to myocardial perfusion imaging, myocardial fatty-acid metabolism can also be observed using 123I-beta-methyl-p-iodophenylpentadecanoic acid (123I-BMIPP). Myocardial fatty-acid metabolism SPECT can visualize myocardial metabolic shift caused by myocardial ischemia and myocardial damage from other causes. This modality is useful for diagnosing relatively-severe myocardial ischemia, such as ACS, and differentiating between ischemic heart disease (IHD) and non-IHD in patients with unknown-cause heart failure [57-60].
3.3 Role of CCTA in cardiac hybrid imaging
CCTA has been recognized as a non-invasive imaging modality for coronary morphology as an alternative to the invasive coronary angiography [61-63]. Recent multi-slice CT scanners can obtain high temporal and spatial resolution images with low radiation exposure [64]. Three multi-center studies have demonstrated the high diagnostic accuracy of 64-slice CCTA with a sensitivity and specificity of 85–99% and 64–90%, respectively. In particular, the high negative predictive value, which is close to 100%, has solidified CCTA as an excellent tool for confirming the absence of CAD in patients with low to intermediate pre-test probability [62,63]. Despite its high diagnostic accuracy, CCTA exclusively shows coronary-artery anatomical findings without hemodynamic information. However, CCTA provides coronary arterial wall findings with information about nonobstructive plaques as well as plaque size, composition, and coronary artery remodeling. The additional prognostic value of detecting non-obstructive coronary plaques has been extensively explored. Motoyama et al. reported that low attenuation plaques, which have CT values less than 30HU, with positive vascular remodeling lead to high incidence of future ACS events [65,66]. According to Otsuka et al., napkin-ring sign shown on CCTA is strongly associated with future ACS events [67-69]. The non-obstructive coronary lesions with these high-risk plaque characteristics, even those without myocardial ischemia, may indicate certain risks of future ACS events. Thus, CCTA can be a complementary modality of myocardial SPECT for cardiac hybrid imaging.
3.4 Cardiac hybrid imaging hardware and software
As it is widely known, hybrid imaging was started with PET/CT hybrid systems, which were mainly used for the field of oncology. Following this advancement of imaging devices, similar hybrid devices, combining SPECT gamma cameras with multi-detector CT scanners, have been recently introduced. These hybrid devices provide location information equally to both SPECT and CT images and enable highly accurate image fusion of these images [70]. However, the majority of these hybrid devices are available with a multi-detector CT device equipped with 16 or fewer detectors despite the recommended minimum number of CT detectors for CCTA being 64. CT devices with a fewer number of detectors have a low temporal and spatial resolution and cannot provide sufficient image quality. As for cardiac hybrid imaging, the use of hybrid systems is not necessarily required. This is due to the myocardium's larger size not requiring the fine detail of hybrid imaging as well as the difference in respiratory conditions between SPECT and CT acquisitions. Whether a hybrid system is used or not, manual correction of misalignment is crucial to improve the quality of fused images because of the following reasons: cardiac and respiratory motion, size and shape difference of the left ventricle due to the difference in spatial resolution, and the presence or absence of ECG-gating. Therefore, cardiac hybrid imaging can easily be performed using stand-alone systems with fusion software. Fusion software packages are available from several manufacturers that allow fusion of CCTA and SPECT images [11]. The image processing steps for creating fusion images usually consist of image co-registration, extraction of LV epicardium, projection of myocardial tracer accumulation to LV epicardium, extraction of coronary tree, and display of 3D volume rendered SPECT/CTA fusion images. Current software packages allow fusion of these images from different manufacturers. Image fusion processing time takes less than 5 minutes for experienced operators, provided the images are high quality.
3.5 Clinical value of cardiac SPECT/CT hybrid imaging
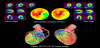
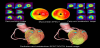
Traditionally, the diagnosis of CAD is conducted using CCTA or myocardial SPECT individually or side-by-side interpretation using both modalities. However, the distribution of coronary arteries differs considerably among individuals and the standardized myocardial segmentation model for coronary artery territory cannot be applied in up to 72% of patients [71]. The largest variation in coronary artery distribution is in the dominance of the right coronary artery or left circumflex artery in the inferoposterior and posterolateral wall of the left ventricular myocardium (Figure 3). Therefore, the additional information provided by cardiac SPECT/CT hybrid imaging can be used in combination with myocardial SPECT and CCTA to ascertain the interpretation of coronary artery territory in different individuals. Furthermore, cardiac SPECT/CT hybrid imaging can show the direct relationships between the distribution of coronary artery stenoses and myocardial ischemia.
The feasibility and clinical demand of non-invasive cardiac SPECT/ CT hybrid imaging, processed using a self-built algorithm, were initially realized by Nakajo et al. [72]. Rispler et al. reported drastic improvement in specificity (from 63 to 95%) and positive predictive value (PPV) (from 31 to 77%) with SPECT/CCTA compared to CCTA alone [73]. Sato et al. showed that supplemental SPECT information, in non-evaluable coronary artery territory on CCTA, significantly improved specificity and PPV (from 80 to 92% and from 69 to 85%, respectively) [74]. However, these studies have several limitations, including the limited number of patients, the variety of hybrid systems, and the lack of a unified reference standard. Three studies have specifically mentioned the additional value of cardiac hybrid imaging in comparisons of myocardial perfusion SPECT and CCTA side-by-side analysis with cardiac SPECT/CT fusion imaging. These reported that the use of cardiac SPECT/CT hybrid imaging decreased the indeterminate diagnostic rate and consequently increased the conclusive diagnostic rate compared to side-by-side interpretations in patients with CAD. These patients, who are often found to have multivessel disease (MVD), moderate coronary stenoses, or branch lesions, also often see the greatest diagnostic improvement in regard to these findings via cardiac SPECT/CT fusion imaging (Figure 4 and 5) [75-77]. Hybrid cardiac imaging can facilitate the detection of hemodynamically significant coronary artery stenosis, leading to appropriate choices of therapeutic methods. Furthermore, confirming hemodynamically non-significant stenosis can avoid unnecessary stent implantations, which may dissipate limited medical resources.
Several studies have revealed the prognostic performance of cardiac SPECT/CT hybrid imaging for patients with CAD. van Werkhoven et al. has reported that significant coronary artery stenoses and noncalcified plaques on CCTA provided additional prognostic value over myocardial perfusion SPECT alone [78]. Furthermore, prognostic information of cardiac SPECT/CT hybrid imaging has been reported by Pazhenkottil, et al. [79]. In this study, 324 consecutive patients were divided into three groups: stenoses by CCTA and matched reversible SPECT defects, unmatched CCTA and SPECT findings, and no findings by CCTA or SPECT. Corresponding matched hybrid image findings were associated with a significantly higher event rate (death or myocardial infarction) (p < 0.005) and proved to be an independent predictor for major adverse cardiac events. The annual event rates were 6.0, 2.8, and 1.3% for patients with matched, unmatched, and no findings, respectively.
Two studies have shown that cardiac hybrid imaging has a strong impact on the choice of therapeutic methods for CAD. Follow-up was confined to the first 60 days after hybrid imaging, as this allows for the best assessment treatment strategy decisions. Pazhenkottil et al. reported that revascularization rates within 60 days were 41, 11, and 0% for matched, unmatched, and no findings, respectively (p < 0.001) [80]. In a similar study by Schaap et al., revascularization rates were even higher, with 90, 31 and 0% for matched, unmatched, and no findings, respectively (p < 0.001) [81]. These results were expanded upon in the SPARC study, which showed 38-61% of patients with the most severe findings on CCTA or myocardial perfusion SPECT did not need invasive coronary angiography [82]. Therefore, non-invasive cardiac hybrid imaging may facilitate optimal patient management for patients with CAD.
3.6 Coronary artery calcium scoring for hybrid imaging
Presence of coronary artery calcification (CAC) is a strong indicator of CAD as well as fatal and non-fatal cardiovascular events [83,84]. CAC is considered an active, regulated process caused by coronary risk factors and consequent plaque inflammation [85]. The amount of coronary artery calcium, which is represented by coronary artery calcium score (CACS), is directly related to the extent of coronary atherosclerotic plaque burden [86]. CAC is usually quantified on dedicated 3 mm sliced, plain, and ECG-gated CT scans [87]. CACS might be more accessible with SPECT/CT hybrid systems, as CT scans with these systems are usually performed with low-radiation exposure and without contrast media. Several studies have shown the increasing prognostic value of CACS above traditional cardiovascular risk factors, suggesting that CACS is a useful clinical marker to predict cardiovascular risk in patients with CAD [88]. Using hybrid imaging, the clinical value of integrating CACS and myocardial perfusion SPECT has been realized.
Schepis et al. presented a comparative investigation between myocardial perfusion SPECT, CACS, and invasive coronary angiography in 77 symptomatic patients with intermediate CAD risk [89]. CACS was closely related to the presence of angiographic CAD and severity of myocardial ischemia. Furthermore, the supplemental use of CACS (the optimal cut-off value of CACS was 709) with myocardial perfusion SPECT increased the sensitivity from 76 to 86% and NPV from 76 to 83% compared to myocardial perfusion SPECT alone. The supplemental use of CACS may help detect multivessel disease in patient’s with pseudonormal myocardial perfusion.
Ghadri et al. presented the adjunct, prognostic value of CACS over myocardial perfusion SPECT using SPECT-CAC hybrid imaging in the REPROSPECT study [90]. Between three groups (matched positive SPECT and CACS findings, unmatched SPECT and CACS findings, and matched negative SPECT and CACS findings), the matched positive group had the most unfavorable outcome (p < 0.001 for MACE and p < 0.01 for death and myocardial infarction).
3.7 Radiation exposure
SPECT and CT are imaging modalities, which entail ionizing radiation exposure. Radiation-induced stochastic effects, such as carcinogenic risk, is directly proportional to radiation dose based on the linear no-threshold model, and unnecessary radiation exposure in patients who undergo medical image procedures should be avoided. Radiation exposure dose remarkably varies depending on imaging modalities, radionuclides, and imaging protocols. A study by Hausleiter et al. reported effective radiation doses in early CCTA with 64-slice CT of up to 21.4 mSv [91]. However, patients’ radiation exposure from the examination has reduced considerably by 30–90% because of new image acquisition protocols, in particular the ECG-gated tube current modulation, body-mass-index-adapted tube voltage modulation, and prospective ECG-triggered sequential scanning [92,93]. The most recent scanning protocols, using iterative reconstruction, have achieved the reduction of dosages to 0.78–0.99 mSv [94].
Similarly, myocardial perfusion SPECT using 201Tl may reach effective radiation doses up to 20–30 mSv [91]. Shorter-half-life perfusion tracers based on 99mTc allow for a much lower radiation exposure of approximately 9.3 mSv for one-day rest and stress tests [91]. The current introduction of solid-state detectors based on CZT may allow for further reduction of radiation exposure due to a higher detector sensitivity and improved energy resolution [95].
Recently, there have been growing concerns about radiation exposure in medical imaging tests due to the increase of CT scans in the clinical setting [4]. The reduction of radiation exposure must be balanced with maintaining a high level of image quality and diagnostic performance. Particularly, in the field of cardiac non-invasive imaging, drastic reductions of radiation exposure have been achieved through optimized image acquisition protocols and advanced imaging devices, thus facilitating the use of hybrid imaging in the clinical setting [92]. A recent report has documented the feasibility of a stress-only SPECT/CCTA hybrid imaging test with a cumulative radiation dose of 5.4 mSv [96]. Recently, tests to determine maximal dose reduction without compromising image quality were carried out and concluded that ultra-low dose (< 190 MBq) myocardial perfusion imaging with short imaging times (< 6 min) is feasible using a hybrid CZT SPECT/ CT camera without compromising image quality [97].
3.8 Clinical indications for hybrid imaging
For diagnosing CAD, either CCTA or myocardial perfusion SPECT is usually chosen for the initial imaging test. As an initial imaging test, CCTA can be chosen for patients with low to intermediate pretest probability and myocardial perfusion SPECT can be chosen for patients with a slightly higher pre-test probability. The presence or absence of CAD can be confirmed with a single non-invasive modality in a large segment of patients and a second non-invasive test may be performed if the first test was inconclusive or equivocal. In addition, myocardial perfusion SPECT is commonly performed on high pre-test probability patients to locate ischemia. However, radiation exposure and examination costs will each increase with the use of both morphological and functional imaging. Therefore, hybrid imaging testing should be carefully considered, and used only if additional value of the imaging is highly expected.
3.9 Intermediate pre-test likelihood
Patients with intermediate pretest likelihood are likely to have inconclusive results in the first non-invasive imaging; these results are usually moderate stenoses in CCTA and equivocal accumulation defects in myocardial SPECT [98]. In this situation, additional imaging may be instrumental to confirm the diagnosis. In patients with moderate coronary stenoses in CCTA, additional myocardial SPECT can prove whether the stenotic lesion is hemodynamically significant or not. In patients with equivocal accumulation defects in myocardial SPECT, additional CCTA can facilitate a diagnosis, which is usually CAD, non-ischemic heart disease, or artifacts. In both cases, sequential hybrid imaging can be of assistance to formulate therapeutic strategies.
3.10 Side branch lesions
While side branches are often regarded as prognostically insignificant, they often have variations in their size and distribution, which can aid in prognosis. In the case of side branches being unusually large, side branch lesions can cause significant myocardial ischemia. The myocardial ischemia caused by side branch lesions may be difficult to distinguish from that caused by main branches: especially, myocardial ischemia caused by a diagonal branch stenosis, which might be mistaken as that caused by a left anterior descending artery stenosis (Figure 4). Cardiac SPECT/CT hybrid imaging provides accurate coregistration of coronary arteries and perfusion defects and hence allows detection of hemodynamically significant side branch lesions which are targets of revascularization [75].
3.11 Multivessel disease
Patients with MVD have an increased risk for future cardiac events and thus are more likely to undergo revascularization to improve their prognosis. CCTA has revealed that patients with MVD account for a significant portion of those with CAD. Since the majority of patients with three-vessel disease often have less flow-limiting lesions, cardiac hybrid imaging may be advantageous to detect hemodynamically significant coronary artery stenoses (Figure 5) [76,99]. As described above, cardiac hybrid imaging may allow for selective revascularization for hemodynamically significant lesions in patients with MVD, avoiding overtreatments with revascularization that may cause adverse sequelae.
3.12 Chronic total occlusions
The implementation of PCI for patients with chronic total occlusions (CTO) is usually associated with a high probability of complications and an increase in the dose of contrast media and radiation exposure. Therefore, myocardial ischemia in the territory of occluded coronary arteries should be proven before PCI. Cardiac hybrid imaging can visualize the morphological information of the lesions, such as the length of the lesion, degree of calcification, and collateral formation, and accurately detect myocardial ischemia in the territory of occluded coronary arteries. Comprehension of the lesion condition prior to PCI might be helpful to improve the procedural success rate [100].
3.13 Post coronary artery bypass grafting
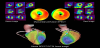
Coronary artery bypass grafting (CABG) is an effective revascularization procedure for patients with advanced CAD. However, graft occlusion or the development of stenoses in the native coronary artery and consequent myocardial ischemia can occur in some cases after the operation (Figure 6) [101,102]. In patients who underwent CABG, multiple coronary stenoses often exist, and the hemodynamic status of the entire coronary artery might be complicated. Since the number of patients with a history of CABG and an indication for revascularization procedures, such as PCI or additional CABG, has been steadily increasing, cardiac SPECT/CT hybrid imaging may play an important role in determining therapeutic strategies [103].
3.14 Differential diagnosis in patients with unknown-cause heart failure
In patients with unknown-cause heart failure, ascertaining the cause is crucial to determine the therapeutic strategy. Myocardial perfusion SPECT can confirm myocardial ischemia and myocardial fatty-acid metabolism SPECT can reveal myocardial damage of any various cause, such as takotsubo cardiomyopathy, cardiac sarcoidosis, and hypertrophic cardiomyopathy [60,104]. In the case of multiple accumulation defects in myocardial SPECT images, differentiating between IHD and non-IHD is often difficult. However, cardiac SPECT/CCTA fused images can show the distribution of coronary arteries and accumulation defects, which can be instrumental to distinguish the diagnoses of IHD and non-IHD (Figure 7) [105].
4. Conclusion
Cardiac SPECT/CT hybrid imaging is facilitated by the dissemination of state-of-the-art devices of nuclear medicine and CT, as well as by SPECT/CT hybrid systems and dedicated software. The combined approach of SPECT and CT has various advantages when compared with stand-alone imaging. CTAC can correct attenuation artifacts in myocardial SPECT and can improve its diagnostic accuracy. As for cardiac SPECT/CT hybrid imaging, a comprehensive approach, with morphological and functional assessment, may enhance diagnostic ability, improve risk stratification, and guide management strategies for patients with CAD. These abilities of cardiac SPECT/CT imaging will be further refined validating a range of clinical applications in large-scale clinical trials.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
The author substantially contributed to the literature review, drafting the manuscript and approve the final version of the manuscript.