1. Introduction
Boron, the fifth element in the periodic table, does not occur in nature in its elemental form. Rather, boron combines with oxygen as a salt or ester of boric acid. There are more than 200 minerals that contain boric oxide but relatively few that are of commercial significance [1]. Boron minerals are differently named regarding to their alkali, earth alkali, boron oxide (B2O3) and water contents and crystal structures [2]. In fact, three minerals represent almost 90% of the borates used by industry: borax (tincal), a sodium borate; ulexite, a sodium–calcium borate; and colemanite, a calcium borate [1].
Turkey is the largest producer of natural borates worldwide. Boron reserves are highly concentrated in Turkey, which accounts for around 70% of the world total. The Turkish borate deposits occur in western Anatolia in five main districts; Bigadic, Kestelek, Sultancayiri, Emet, and Kirka. The borates in Turkey are tincal, colemanite and ulexite. Colemanite reserves have the largest share (73.9%) compared to tincal (24.5%) and ulexite (1.6%). Colemanite and tincal are the most widely-used boron minerals. B2O3 wt. percentages of the minerals are 50.8% and 36.5%, respectively. Nearly 10% of the natural borates are consumed as it is; whereas the rest is used to produce refined products [3].
Boron oxide-bearing minerals can technologically be considered as important alternative raw materials since they have a glass-forming character and have been used in the ceramic industry to reduce the melting point, lower the viscosity and decrease the CLTE of the glass phase and therefore raise its heat-resistance, and increase the mechanical strength and chemical stability of the materials [4,5]. Presently, borates, especially colemanite, find a wide variety of applications in industry [6]. Colemanite is a hydrated calcium borate Ca2B6O11.5H2O with crystalline structure of complex chains of tetrahedral B(O,OH)4 and triangles BO3, bound into a triangular structure via the ions Ca2+ and buffer molecules H2O [5]. Colemanite is an industrially important mineral, which is mostly used in textile type fiberglass, glass and ceramic industries and metallurgy. Colemanite is also used in detergent and cosmetic industries and for production of boric acid by reacting with sulfuric acid. When used in textile type fiberglass industry, colemanite drops the melting temperature of the mixture, provides low viscosity at the melting temperature, prevents crystallization and positively affects the chemical and physical properties of final glass product. Colemanite reduces the melting point in glass industry and it is resistant to thermal shocks. In formulation of ceramic and enamel glazes, it provides a stable structure, homogeneous melting and low segregation. Since it is a solvent for almost all metal oxides, it is used as a fluxing agent in the metallurgy industry [7].
When boron minerals are heated, the mineral first loses water of crystallization, followed by production of amorphous material or recrystallization into new phases [8]. The decomposition process of colemanite mineral exhibits intra-crystalline thermal dissociation. While formation of the water molecules from the OH groups in the structure take place, water molecules which have been formed by this step, released by the heating energy. Two stages of this dissociation events are proceed uniformly within the crystal grains of the mineral. Waclawska et al. (1998) report that colemanite structure involves enclosed water molecules within the structure and the pressure of the enclosed H2O molecules increases rapidly that cause an explosive water loss with increasing temperature [9]. This sudden release within the micropores induces a disruption of the framework by increment of the temperature [8-9]. For this reason, direct use of colemanite mineral is limited as a raw material in ceramic industry. The other issue is that deformation problems will be observed in the final products if boron containing minerals are used with high amounts in ceramic products when they preferred to densify in fast firing process [10]. Therefore, colemanite mineral should be calcined in order to prevent such problems before usage for sintering aid.
Decomposition of colemanite by conventionally should be performed with very slow heating rates in order to avoid harmful effects of the severe water release within micropores of colemanite. Unfortunately, slow heating rates bring very long processing durations and cause slower production rates and high costs. At this point, microwave heating process is a technology that can provide fast heat generation within the body and shorter process duration. Since microwave heating process provides rapid heating of materials without overheating the surface, removal of water vapour from materials can be smooth without cracking. It is therefore possible to use in calcination process by microwave heating, in a fraction of the time that it takes for conventional heating. The difficulty to coupling microwave and material at low temperatures can be improved with the help of a hybrid heating scheme that combines microwave and convective heating by the use of susceptors to bring the temperature of the material to its critical microwave coupling temperature (microwave assisted heating) [11]. Therefore, it is thought to be an alternative approach use of microwave energy instead of conventional method in the calcination process. The purpose of this research is to compare of both conventional and microwave techniques on the calcination of colemanite and to explore the advantages of microwave heating on the process duration and temperature. If this aim is made possible, then the ceramic industry will benefit by having a new and cheaper raw material.
2. Experimental Procedure
The ground colemanite under 75 micron used in this study was provided from Eti Mine in Turkey. Comparison of both conventional and microwave techniques on the calcination of colemanite were performed. In conventional calcination studies, colemanite samples were heated to 450 and 700°C with a heating rate of 10°C/min. The temperatures were selected in an interval between release of crystal water and the beginning of the amorphization behavior, which are determined by the results of DTA–TG analysis of colemanite powder. In microwave calcination studies, colemanite samples were heated to the same temperatures by using a modified domestic microwave oven with different power levels (385, 539 and 700 W) in order to determine optimal power level in calcination process. Modification of domestic microwave oven described as follows; silicon carbide susceptors were used for initial heating of the samples which are insensitive to microwave radiation. Porous fiberboard cubic box of 10 cm3 was used as thermal insulation (so-called thermal box) to maintain temperature increase for calcination process and also to protect the internal chamber of the microwave oven. Two half-moon shaped SiC susceptors were placed in a symmetrically configuration within the box, and the material to be processed in a crucible is placed in the central position of the susceptors. The entire thermal box was then placed in the chamber of microwave oven for processing. The temperature of the samples was monitored using a thermocouple (TP-01 Type K) setup coupled with a data logger and thermocouple probe was inserted to the center of the crucible. Calcination of colemanite powders were carried out at different power levels as a function of time. By this way, temperature-time profiles for each power levels were observed for the setup.
Thermal behavior of colemanite samples for conventional heating was carried out using a Netzsch STA 449 differential thermal analyzer with a thermogravimetric analyzer (DTA-TG) at 10°C/min in air atmosphere. Thermal behavior of colemanite samples for microwave assisted heating was evaluated by volumetric and mass changes (Figure 1) at different temperatures (450-600°C). X-ray diffraction (XRD) analysis was performed by a Bruker-D8 Advance type X-ray diffractometer using Cu Kα radiation to identify the present crystalline phases in the raw and calcined states of the samples. Characterizations of surface area, pore size and pore volumes of the calcined samples were evaluated by BET (Brunauer-Emmett-Teller) analysis.
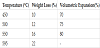
3. Results and Discussion
Temperature vs time graphics for different power levels; medium (385 W), high (539 W), very high (700 W) of modified domestic microwave oven is shown in Figure 2a. Temperature measurements were verified by three different measurements. It can be clearly seen that microwave-assisted heating supply very close temperature fluctuations within the same power groups. According to these data,it can be said that 700W and 539 W microwave power levels require approximately same time to reach the same temperature, whilst 385 W power level needs more. For this reason, high level (539 W) was determined as optimal power level in this study in order to save time as well as energy. Also, microwave-assisted heating can offer significant time savings compared to conventional heating as indicated in Figure 2b. The further studies in microwave calcination process were carried out in the range from 450°C to 700°C; a five minute standing period at these temperatures in high power level (539 Watt). % weight loss (Δm) and % volumetric expansion (Δv) values of calcined colemanite at different temperatures are listed in Table 1 where both Δm and Δv values (%) are trending with the increase of temperature. A volumetric expansion of 70% in the sample at 450°C was observed and it has reached up to 80% with increase in temperature and ended by partial melting of colemanite at 595°C in microwave assisted calcination (Figure 1d).
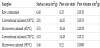
Surface area, pore size and pore volume values of colemanite heat-treated at different temperatures by microwave and conventional heating techniques are presented in Table 2. Elevated temperatures result in reduction of the surface area values of calcined forms. It was also observed that the pore size of the heat-treated samples enlarged compared to raw state of the colemanite. Furthermore, pore size values significantly increased after the calcination by both of microwave and conventional heating techniques at 450°C. Due to softening effect, this value plainly decline with higher calcination temperatures. When the samples calcined at 450°C were compared according to the heating technique, it is clearly seen that microwave-heated samples exhibits lower surface area and higher pore size and pore volume values than those of conventional calcined samples. A volumetric expansion of 70% with the increase in the pore size of calcined sample at 450°C also confirm the pressure of the enclosed water molecules increases so much that it disrupts the framework and they are released.
Thermal analysis of colemanite was studied by many researchers. Waclawska et al. proposed that colemanite decomposes in two mechanisms: (1) formation of H2O from OH groups and (2) breaking of H2O and borate chains bonds and then removal of both kinds of water from the anhydrous phase of the preserved borate structure [9]. DTA curves for raw colemanite samples examined under the standard conditions show two endothermic peaks within the temperature range 325-440°C (Figure 3). The first one is at 384°C and starts a distinct inflection at 360°C. This first event means that the thermal dissociation of colemanite begins with the removal of the OH groups, which form water molecules. Next, the bonds of molecular water with borate rings become broken. This can be judged from the endothermic peak at 398°C. According to Waclawska et al., this endothermic reaction is accompanied by disintegration of the sample into small particles and their spraying [9]. In addition, heat microscopy studies by Wacklawska have shown that this process has a sudden and explosive character [9]. Next, the removal of water proceeds rapidly up to approximately 440°C as observed from the TG curve. The peaks at 384°C and 398°C are accompanied by a 10.23 wt.% loss due to the release of water. The water content in the colemanite sample examined lower than that of theoretically calculated (21.84 wt. %) is the result of the presence of calcite in its crystal structure as will be seen in XRD results below. The remaining water release slowly up to 600°C. At a temperature of 751°C there occurs an exothermic peak accompanied by change of 4.8 wt.% in the weight of the sample, preceded by a weak endothermic peak (662°C). Characteristics of DTA trace indicates that it is caused by an exothermic crystallization (751°C) below the melting point of the compound (950°C). These observations are comparable with those of Waclawska [9].
A weight loss of 10% in the sample at 450°C observed in the microwave calcination (Table 1) was found to overlap with the weight loss at the same temperature in the DTA-TG analysis. Besides, the weight loss is determined as 23% in the DTA-TG analysis until 800°C was found to occur in the microwave-assisted calcination up to 595°C.
To understand the nature of the processes that have occurred after heating, an X-ray analysis of the raw colemanite and the colemanite samples heat-treated at different temperatures were performed (Figure 4). The investigations showed that the initial raw form consists primarily of the mineral colemanite with a significant amount of calcite (CaCO3). Moreover, during heat treatment, the structure of colemanite is exposed to a series of transformations including dehydroxylation and dehydration up to 450°C as seen in DTG analysis curve. It is seen that this disintegration reaction is accompanied by approximately 10 wt. % loss in TG analysis. It is understood that disintegration of colemanite occurred also in microwave heating through the similar weight loss was observed by conventional calcination at the same temperature. Furthermore, Table 2 shows that the pore size value by both heating routes at this temperature has a considerable change and there is an enlargement in the pore size by disintegration reaction. According to our experiences the XRD patterns of powders after conventional and microwave-assisted calcination at 450°C is crucial.
At the stated temperature, although a significant change at phase structure was not observed compared with raw material, a decrease at peak intensity was determined. This supports that colemanite forms only decomposition reaction with the conventional and microwaveassisted calcination at 450°C. It was seen in Figure 1d that at 595°C in microwave-assisted calcination, colemanite begins to melt partially and powders change into the compact state through hardening. For the XRD analysis made at that temperature it is observed that peaks belonging to colemanite lose almost completely and only the peaks belonging to calcite remain, phase structure changes with the calcination at the stated conditions. It can be understood that colemanite is decomposed to B2O3 and CaO in their amorphous
forms and calcite upon calcination. As a result of the observations, it is determined that partial melting occurs at 700°C at conventional heating process while it occurs at 595°C by microwave assisted heating. It is found out that XRD investigations at starting temperature of partial melting by both heating routes are similar. This situation shows that microwave assisted calcination provides an advantage as about 100°C decrease at the transition temperature of colemanite into amorphous calcium borate and crystal calcite.
During the preliminary experiments of this research performed without susceptors and under direct microwave heating, thermal runaway characteristics of colemanite was observed at 539 W for 10 minute condition as material fully liquidified in crucible. Therefore our systematic studies with susceptors system revealed that the shortest period of calcination is performed at 450°C for 10 minutes periods which is obviously 100°C lower that melting point.
4. Conclusion
The advantages of microwave heating on the process period and temperature was investigated through both convetional and microwave techniques for calcination of colemanite. Structural properties of as received colemanite and heat treated powders were characterized by XRD, DTA-TG and BET analyses and thermal behavior of colemanite for microwave assisted heating was evaluated by volumetric and mass changes. Besides the major colemanite mineral, calcite also the other main mineral in the powder was detected in XRD analysis. During the decomposition of colemanite, the OH groups are the first to break off from the borate anions (dehydroxylation) and the water molecules split off (dehydration) within the temperature interval 325-440°C. A volumetric expansion of 70% with the increase in the pore size of the calcined sample at 450°C confirm the pressure of the enclosed water molecules increases so much that it disrupts the framework and they are released. Characterization results are revealed that microwave assisted calcinations provides an advantage as process time the lowest calcination temperature to be 450°C as 10 minutes and in terms of a 100 °C reduction of the transition temperature of colemanite into amorphous calcium borate and crystal calcite.
Competing Interests
The authors declare that they have no competing interests.