1. Introduction
Serum alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) are recognized as useful markers for the diagnosis of hepatocellular carcinoma (HCC). Although 1.3%–15% of all gastric carcinomas produce AFP [1], gastric carcinomas accompanied by elevated serum levels of both AFP and DCP are rare [4-12]. The diagnostic criteria for gastric carcinoma producing AFP and DCP are currently unclear. Among cases affected by such carcinomas, immunohistochemical staining of DCP in two cases in the literature was paradoxically positive, indicating gastric carcinoma. Here, we report a patient with gastric carcinoma with abnormally high levels of AFP and DCP in serum and only DCP expressed by liver metastases.
2. Case Report
A 77-year-old man visited our hospital because of watery diarrhea for 3 days and abdominal discomfort. He had no history of blood transfusion and alcohol abuse. Physical examination revealed an elastic and firm liver that was palpable 8 cm below the right costal margin.
Initial laboratory tests revealed the following levels: serum aspartate aminotransferase, 74 IU/L (normal, <38 IU/L); serum alanine aminotransferase, 47 IU/L (normal, <44 IU/L); serum alkaline phosphatase, 2447 IU/L (normal, 104–338 IU/L); serum lactic dehydrogenase, 449 IU/L (normal, 180–460 IU/L); and total serum bilirubin, 1.7 mg/dl (normal, 0.2–1.2 mg/dl). The serum DCP level was 13,500 mAU/mL (normal, <40 mAU/mL), and the serum AFP level was 187,800 ng/mL (normal, <6.92 ng/mL). Furthermore, the lectin-reactive AFP-L3 fraction was 48%, serum carcinoembryonic antigen (CEA) concentration was 8.9 ng/mL (normal, <2.5 ng/ mL), and CA 19-9 concentration was 27.2 U/mL (normal, <37 U/ mL). Serum hepatitis B surface antigen and antibodies to hepatitis B and hepatitis C were not detected. Laboratory tests showed that the hemoglobin level was 11.1 g/dL and that the prothrombin time was 95.9% (international normalized ratio, 1.03).
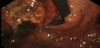
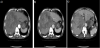
Upper gastrointestinal endoscopy revealed a longitudinal, 5-cm long, advanced gastric carcinoma (type 2) at the cardia (Figure 1). An abdominal computed tomography (CT) showed multiple low-density areas in the liver, and the largest tumor was 13 cm in diameter. The tumor was enhanced at the peripheral zone, mosaic inside in the early phase, and washed out in the late phase. A bulky gastric lymph node was detected at the right side of the tumor (Figure 2).
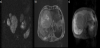
Contrast magnetic resonance imaging (MRI) showed that multiple nodules exhibited low signal intensity on T1-weighted imaging (T1WI) and high intensity on T2-weighted imaging (T2WI). The nodule inside showed high signal intensity on T2WI and diffusion-weighted imaging (Figure 3a). The tumor was enhanced heterogeneously in the early phase and washed out in the late to hepatocellular phase (Figure 3b, c).
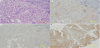
Gastric biopsy confirmed the diagnosis of fetal intestinal adenocarcinoma. The cancer cells were irregular tubular and atypical alveolar epithelial cells with a clear reticulum (Figure 4a). Immunohistochemical staining revealed that the tumor cells expressed AFP (Figure 4b) but not DCP (Figure 4c). Liver biopsy showed that the tumor cells showed basophilic reticulum and anisokaryosis, had extended oval nuclei, and had a structure forming a solid alveolar sheet surrounding the stroma of the vascular connective tissue. Histopathological findings were similar to the gastric biopsy specimen; however, the liver cancer cells expressed DCP (Figure 4d). Therefore, we used the oral fluoropyrimidine anticancer drug TS-1 (80 mg/kg of body weight) to treat the patient. One month after admission, the patient experienced cardiopulmonary arrest owing to massive hematemesis.
3. Discussion
Gastric carcinomas expressing both AFP and DCP are extremely rare and difficult to diagnose and treat [4-12]. In our patient, both the serum AFP and DCP levels were extremely high, the serum CEA level was slightly elevated, and the CA 19-9 level was normal. Multiple liver nodules were observed on CT. In addition, immunohistopathology revealed that the advanced gastric carcinoma contained tumor cells that showed partly positive AFP staining. For the liver tumor, CT showed an HCC-like lesion in contrast to the metastasis shown by MRI. Differential diagnosis of the liver tumor to confirm whether it was a metastatic tumor and primary HCC was difficult; the reason for the extremely high concentration of DCP was unclear.
The complication rate of the liver biopsy was low [2]. To confirm the diagnosis, we performed a needle liver biopsy under ultrasound guidance, and histopathological analysis showed a close similarity to the gastric lesion. In contrast to the stomach lesion, the liver lesion was positive for DCP on histopathological analysis. These findings clinically confirm that the patient had gastric carcinoma expressing AFP and DCP and that the tumor had metastasized to the liver.
Compared to ordinary type 2 and 3 gastric carcinoma at an advanced stage, primary AFP-producing gastric carcinomas have been associated with a higher incidence of concomitant lymph node metastasis, lymphatic, and venous invasion in the gastric wall; liver metastasis; lower radical respectability rates; and a worse prognosis. Liver metastasis occurs in 70%–80% of cases of AFP-producing gastric carcinomas, and approximately half of them are metachronous, with significant elevation in the serum AFP level that is detectable before the lesions are identified by imaging [16]. Furthermore, the AFPproducing tumor showed central necrosis, which was a useful finding for differential diagnosis of the liver tumor.
AFP-producing gastric carcinoma can be divided into 3 subtypes, namely, hepatoid type, yolk sac tumor-like type, and fetal gastrointestinal type [17]. The hepatoid type is the most common and highly malignant [17,18]. AFP-producing gastric carcinomas have a higher malignant potential including higher proliferative activity, reduced apoptosis, and more neovascularization than AFP-negative gastric carcinomas. These biological characteristics of AFP-producing gastric carcinomas result in aggressive behavior and a poor prognosis [19]. In addition, increased c-Met expression contributes to the poor prognosis of patients with AFP-producing gastric carcinoma [20]. However, we did not find any cases of AFP-producing gastric carcinoma with an extremely high level of DCP in the literature.
DCP is a specific marker of HCC and an independent tumor marker that is not associated with AFP [21,22]. In addition to HCC, elevated DCP levels can be found in other metastatic liver cancers, complicating the differential diagnosis between HCC and metastasis. In general, vitamin K deficiency and warfarin intake can induce an increase in serum DCP levels and serum prothrombin time. A histopathological feature of DCP-producing tumors is the presence of an AFP-producing hepatoid gastric adenocarcinoma [4]. Histopathologically, the AFPproducing gastric carcinoma showed areas of tubular adenocarcinoma and hepatoid cells. The yolk sac type of AFP-producing gastric carcinoma showed the presence of tubular adenocarcinoma and yolk sac tumor cells. Immunohistochemically, almost all the yolk sac tumor cells were positive for AFP. The ability of tumor cells to produce AFP seemed to be attributed to retrodifferentiation of adenocarcinoma cells [3] or transdifferentiation to hepatocellular metaplasia. Similarly, a part of the retrodifferentiated adenocarcinoma cells gained the ability to produce DCP. In our case, the gastric lesion was negative for DCP but the liver lesion was positive, suggesting that the tumor gained the ability to produce DCP during metastatic progression into the liver, which was accompanied by transdifferentiation, or that the gastric biopsy specimen did not contain sufficient tumor cells to indicate DCP positivity immunohistochemically.
4. Conclusions
Although the gastric lesion in the current patient did not show DCP positivity, the patient was diagnosed with AFP- and DCPproducing gastric carcinoma. Gastric carcinomas producing AFP and DCP are considered as high-grade malignancies because they tend to metastasize to the liver and lymph node in spite of macroscopic detection through upper gastrointestinal endoscopy in the early stage. The therapeutic approach rages from resected liver metastases including primary gastric tumor to best supportive care. However, there are only a few reported cases of long-term survival associated with high-grade malignancy. In the present case, survival time was short and a satisfactory treatment outcome was difficult to achieve. In many cases, chemotherapy is enforced as the first-line treatment, because many cases progress to an advanced stage. As such, the therapeutic strategy is not definite. Moreover, although cases of positive response to chemotherapy have been reported in gastric carcinomas producing AFP and DCP,. n the present case, short time outcome was induced in chemotherapy, because the treatment course of gastric carcinoma with extremely high concentration of serum AFP and DCP with multiple liver tumors seemed to be BSC in consideration of AFP and DCP producing gastric carcinoma.
Competing Interests
The author(s) declare that they have no competing interests.