1. Introduction
Candida albicans inhabits the gastrointestinal tract as a typical dormant commensal member but can become an opportunistic pathogen when the host microflora is compromised [1]. Candida infections (candidiasis) can be life threatening, particularly in individuals who are critically ill, (immunodeficiency syndrome, hematological malignancy) causing mortality rates of over 30-40% [2]. Adhesion to a surface, whether mammalian or synthetic, is the first step in its pathogenic phase followed by a morphological change from the yeast to hyphae phenotype (the virulent state) [3].
Moreover, C. albicans biofilm formation is becoming a common occurrence on catheters and other types of intravenous devices, which if not surgically replaced, can lead to life threatening systemic infections [4,5]. The estimated costs of treating such infections are exceeding a billion dollars a year in the United States alone [6]. The increasing C. albicans infection rate has been attributed to the emergence of resistant strains to commonly used antifungal agents. Moreover, considering that infectious diseases have are causing ever increasing mortality rate among human population, pathogens appear to have a greater ability to transform and attain resistance to antimicrobial drugs [7,8] thereby necessitating the development of innovative and multiple targeted anti-fungal agents.
Phenolic compounds present in grasses (such as supina) have may exert such anti-fungal properties due to their ability to complex proteins, disrupt microbial membranes or act as cell signaling agents [9,10]. In fact, as secondary components in plants, these compounds function as antifungal and antibacterial to protect a natural system. As such, plants have been used to treat and prevent diseases over thousands of years. Reports have shown the health promoting benefits of even though their mechanisms modes of actions are not fully understood [11]. However, such research will require an interdisciplinary approach due to the rich levels of phenols present in our environment coupled with their chemical diversity, which in turn affects their solubility, stability, desolation and absorption, which all constitutes their release that potentially affects antimicrobial potency [12]. Additionally, as phenolic compounds are largely available in all of plants, and thus typically consumed as a part of large matrix in our diets, it is a challenge to identify their specific health promoting benefits, but have properties that protect against cellular oxidation, cellular inflammation, energy dysfunctions, cancer, heart disease, diabetes, to name a few of the studies conducted thus far. In particular, phenols have been effectively used as antimicrobials in multiple studies [13-19]. Although, most of these studies have been conducted in with isolated phenols, humans consume a richly diverse composition of phenols on a daily basis via plant-based food intake. As such, it is only reasonable to hypothesize that phenols from plants act together to provide their health benefits, and thus the development of novel drugs from these components should take this approach [20].
Moreover, several type of plant extracts demonstrated antimicrobial activity against candida including Curcuma zedoaria, Psidium guajava, Plectranthus amboinicus, Aristolochia cymbifera, Plectranthus barbatus, Lippia alba, Hydrocotyle bonariensis, Hydrocotyle bonariensis, Justicia pectoralis var. stenophylla, Herreria salsaparilha, Mentha X piperita, Eleutherine bulbosa, Baccharis trimera, Calamintha adscendens, Albizia inundata, Bauhinia forficata, Cymbopogon citratus, Plectranthus grandis, and Euphorbia hirta L. [15-18]. A study by Polaquini et al. [21] showed the effect of a crude extract of Neem (Azadirachta indica), on inhibiting Candida adhesion, however, it did discuss Candida biofilm formation inhibition. Crossandra infundibuliformis and Labisia pumila Benth extracts also demonstrated potential in inhibiting Candida spp growth and filamentous antifungal activity [22,23]. The extracts also showed from Bauhinia racemosa Lam showed antimicrobial activity against Candida albicans [19].
A study demonstrated a high anti-adherent potential of (S. terebinthifolius, C.urucurana) extracts on in vitro C. albicans biofilm formation [24]. The dried bark of Acacia catechu (family: leguminosae, sub family: mimosaceae) was also able to suppress microbial growth and enhance the immune system to face the invaded antigens of organisms. Acacia catechu have shown potency as antimicrobial agent due to its Taxifolin and active chemical ingredients including (catechin, epicatechin, epigallocatechin, epicatechin gallate, and quercetin [25].
Strawberry, raspberry, and cloudberry extracts demonstrated potential effect on suppress C. albicans growth [26]. Another study investigated the effect of Propolis, C. albicans virulence factors. Propolis is a collective substance from plant source by honeybee, that showed dramatic reduction of C. albicans adhesion, yeast-mycelial conversion, and hyphae length at 0.22 mg/ml [27].
Common to these studies is the use of plant extracts, which all contain phenolic compounds and chlorophyll. Phenolic and flavonoids compounds have been associated with the potential of plants extracts to act as antimicrobial agents. In a study by Rauha et al. [28], reported that purple loosestrife (Lythrum salicaria L plant extracts was very active against Candida albicans, while white birch (Betula pubescens Ehrh.), pine (Pinus sylvestris L.) and potato (Solanum tuberosum. L.) plant extracts significantly inhibited gram-positive Staphylococcus aureus. However, although grasses are the most abundant plant in the world, studies related to their ability to protect against C. albicans virulent factors, among other health promoting benefits, are nonexistence to our knowledge, which has made this grass species one most underutilized agriproduct in modern medicine [29].
The Poaceae grass family is among the most abundant and renewable plant families on the planet that may offer a novel source of phenols [30-32]. The cereal species in particular (corn, rice, and wheat) are staples food that are widely consumed on a global basis, and these species have been recognized as the primary nutraceutical sources within the Poaceae family [31]. However, Poa supina grass is an interesting species among grass family due to its turf characteristics and it is a native species to the European Alps [33]. Despite its potential to contain chemically diverse phenols, the spina grass typically disposed of in the landfills as grass clippings. Therefore, the objective of this project is to determine the ability of a supina grass extract to remediate C. albicans (A2 and SC5314) and possible synergistic interactions of the phenols present in this plant. Thus, an extract of supina grass was characterized for the presence of multiple components, including the phenols, to provide a point of reference of it composition pending a positive impact. Moreover, supina grass was selected as it easy to cultivate in short time spam time thereby providing sustainability as a source of extractable phenols from a drug related perspective. Also, natural plant extracts have demonstrated a superior metabolic power, which might be attributed to the balance phenolics present in plant extracts [34]. As a result, it is expected that this project will provide information on whether this sustainable coproduct stream could be a source of anti-fungal phenol synergists and if a more complex matrix of enriched phenols is more effective in targeting C. albicans virulence phenotypes.
2. Materials and Methods
2.1 Supina grass extraction
Supina grass was provided by Dr. Roch Gaussoin from Agronomy and Horticulture department - University of Nebraska Lincoln, Clippings of the grass were sequentially extracted with (25:75 and 75:25 water: methanol and ethanol) using the same pellet after each extraction in order to recover the chemically diverse polyphenols. Approximately (0.5 g) of finely ground supina grass was extracted with (10 ml) of a solvent for ~ 1 h and centrifuged for 15 min. The supernatant was collected, and the pelleted residue was extracted with the next solvent. Each supernatant was analyzed for total phenols, flavonoids, anthocyanins, chlorophyll, and then the values were added to obtain the final concentrations.
2.2 Total phenols
Total phenolics content of supina grass extract was determined by Folin-Ciocalteu method [35]. Extract aliquots (100 μl) were treated with (100 μl) Folin-Ciocalteu reagent and (4.5 ml) of nanopore water. After 3 min of mixing, (0.3 ml) of 2% (w/v) sodium carbonate was added and the samples were incubated at room temperature for 2h with intermittent shaking. The absorption at wavelength 760 nm was measured with a Beckmen Coulter DU 800 Spectrophotometer (Fullerton, CA). The samples were expressed as the means +/- standard deviation calculated to mg gallic acid equivalent g-1 GT (dry weight).
2.3 Total flavonoids
Total flavonoids were determined by the method according to Adom and Liu [36]. Extracts (125 μl) with proper dilution was added to (37.5 μl) of 5 % (w/v) sodium nitrite and (0.625 ml) of nanopore water. After 4-6 min of incubation at room temperature, (75 μL) of 10 % (w/v) aluminum chloride was added to the sample. Following an additional 5-7 min of incubation, (0.25 ml) of (1.0 M) sodium hydroxide and (0.4 ml) nanopore water was added to the mixture. The samples then were vortexed and monitored at 510 nm/ total flavonoids was expressed as mg catechin equivalents g-1 grass clipping (dry weight) of triplicate analyses.
2.4 HPLC profile
The extracts were hydrolyzed using Devananad et al. [37] by weighing (200 mg) of sample into 50 ml conical plastic tubes. 2 N Sodium hydroxide (2 M) was added (5 mL) in water containing (910 mM EDTA and 1% ascorbic acid) followed by throughout mixing. After the mixture was stirred for 30 min at 40 to 45 °C, 1.4 ml of 7.2 N hydrochloric acid in water were added and vortexed for 10 s. The free phenolics in the were extracted from the samples by adding (6.4 mL) of ethyl acetate and centrifuged at until a clear supernatant was obtained. The organic layers were transferred to another (50 ml) tube, and the extraction was repeated again, and the extracts combined. The extracts were dried under a steady stream of liquid nitrogen until the residue was completely dried, which were then diluted in 1 ml of methanol: water (80:20), vortexed for 3 time at 30 s per vortex to dissolve the residue. The samples were filtered through PVDF syringe filter (0.45 μm) and analyzed by HPLC.
Phenolic profiling was completed on extract that show high potency by using a reverse-phase HPLC system coupled with a C18 (5 μm, 250 x 4.6 mm) and a photo-diode array detector. The method reported by Lin et al. [38] was adopted for resolving the phenolic acids. In brief, the mobile phase consisted of a combination of A (0.1% formic acid in water) and B (acetonitrile) with a flowrate of 1 ml/min. The gradient was varied linearly from (10-26% B (v/v) in 40 min, to 65% B at 70 min, and finally to 100% B at 71 min and held at 100% B to 75 min using a flowrate of 1 ml/min). The UV-vis spectra from (190 to 650 nm) was collected using a photodiode array detector. The resolved peaks were identified and quantified with external standards and expressed as the mean +/- standard deviation of μg per g of clipped grass for triplicate analyses.
2.5 Preparation of C.albicans yeast stock culture
C. albicans strains (SC5314 and A72) were obtained from Kenneth Nickerson, University of Nebraska - Lincoln. A stock culture was grown to the stationary phase. i.e., no visible budding was observed which was typically 24 -30 h post inoculation, in (500 ml) of yeast extract (5 g) -peptone (2.5 g) -dextrose (10 g) medium (YPD). The media (25 ml) was then be added to (125 ml) Erlenmeyer flasks, and a ½ loopful of C. albicans (A72 and SC5314) had been maintained on YPD agar. The inoculated flasks were incubated in a shaking water bath at 30 °C for 22 h (or until cells achieved stationary phase, i.e., no visible signs of budding). The cells were then washed three times with potassium phosphate buffer (pH 6.5) followed each time with centrifugation until a clear supernatant was obtained. The ensuing pellet was then re-suspended in (7.5 ml) of PBS and maintained at 8-10 °C until use.
2.6 Virulent cell induction and treatment
Serum media from (Atlanta Biological) was thawed at room temperature for 5 min, and then (5 ml) of the serum was dissolved in (45 ml) of potassium phosphate buffer (pH 6.5) prior to use. The (25-75 and 75-25 methanol and ethanol) extracts were combined, and (1.5 ml) was concentrated under a steady steam liquid nitrogen. After the sample was completely dried, the residue was dissolved in (1 ml of 100% ethanol) as a stock solution. Different concentrations (0.72, 7.2, 72,720, and 7200 ng/g) of the extracts were prepared by re-diluting in (2%) ethanol. The stock solution þ-coumaric and ferulic acids were prepared by preparing (300 mM) of each phenol in (100%) of ethanol until the solids had completely dissolved. Then, the concentrations ranging from (0.03, 0.06, 0.125, 0.25, 0.5, 1, and 3 mM) were prepared by re-diluting the stock solution into (2%) ethanol. Preliminary experiments were completed that showed an organic solvent was needed to ensure complete solvation of the phenols. Methanol at any concentration affected the adhesion and biofilm formation assays, while ethanol at concentrations below (2%) did not hinder induction of the two virulent factors (data not shown).
2.7 Remediation experiments
C. albicans strains (A72 and SC5314) were added at (5 x 106 cell per ml) in each well of Immunol 2HB 96 well plates containing (140 μl) serum. The plates were covered with aluminum and incubated at 37 °C. After the 24 h incubation, (60 μl) of the extracts at a given concentration that ranged from (0.72-7200 ng/g) were added to each well. The plates were covered again with aluminum and incubated at 37 °C for 6 h. Adhesions and remediation studies were analyzed at (1, 3, 6 and 24 h). The 6 h point was selected for further analysis as preliminary studies showed cellular adhesions and optimal biofilm formation occurred at this time point.
2.8 Biofilm assay
Biofilm formation was determined by using the (2,3-Bis-(2- Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide) XTT kit according to the manufacturer’s direction (Sigma-Alorich). (The XTT kit consists of XTT labeling reagent and electron coupling reagent. This assay relies on yellow tetra zoluim XTT salt cleavage to an orange formazan through the active metabolic cells, which indicating the viable cells, which are based on procedures cited by Pierce et al. [39] & Sudjana et al. [40]. The XTT labeling reagent and electron coupling reagent were thawed in a water bath set at 37 °C, and then (0.1 ml) of electron coupling reagent was added to (5 ml) of XTT labeling reagent to be activated prior to use. The XTT mixture (100 μl) was added to each well and incubated for 2 h. The absorbance was determined at 450 nm using a microtiter plate reader.
2.9 Percent remediation calculations
Remediation of biofilm formation or C. albicans adhesion already established films were defined as % remediation for both cases, which was determined by the following equation [41]:
Where: Acontrol is the absorbance of cells without a treatment
Asample is the cells with the treatment.
2.10 Statistical analysis
The biofilm/adhesion experiments were completed on 3-9 replicates for each treatment /concentrate used and the time point monitored. After data outliers were removed by the Grubs test at a 5% confidence interval, the final results were reported as the mean +/- standard deviation of the One-way ANOVA was used to determine whether that various treatments differed in terms of % remediation at 95% confidence interval (p < 0.05) using Tukey’s honest significant difference. Supina grass clipping characterization analyses were completed in triplicate and the results expressed as the mean +/- standard deviation. The statistical analyses were obtained with Minitab 17.
3. Results and Discussion
3.1 Characterization of supina grass
3.1.1 Total phenols
Phenolic compounds such as, ferulic, caffeic, p-hydroxybenzoic, protocatechuic, p-coumaric, vanillic, and synergic acids are typically present cereal grains [42]. Theses phenolics are present as conjugates, bind with sugars, fatty acids, or proteins [43]. In this work, total phenols of supina grass extracts were quantified by Folin-Ciocalteu method. The result indicated that total phenols of supina grass extracts was 0.96 ± 0.05 mg/g shown in (Table 1). In a study by Wenger [29], the total phenolic content of supina grass was 0.89 ± 13 mg/g. These differences may be attributed to the extraction methods used as preliminary data shown in our lab have shown that even the solid to water ratio substantially affects the phenolic level.
3.1.2 Total flavonoids
Flavonoids are polyphenolic compounds that also abundant in the plan kingdom, and mainly include flavanols, flavanols, and anthocyanins [9]. They are known for several biological activities with an emphasis on their antioxidant properties as they can scavenge free radical, chelate reactive metals, activate the activity of antioxidative enzymes, such as super oxidase dehydrogenase, or inhibit pro-oxidant enzymes, such as xanthine oxidase [44-46]. Flavonoids to possess health promoting properties considered a health promoter, which have the potential to prevent or treat human chronic diseases [46]. There is a growing interest of flavonoids antipathogenic properties due to the increasing resistance of pathogens to available drugs [44]. Therefore, in this study total flavonoids present in supina grass extracts were determined and the results showed that extracts contain 0.49 ± 0.01 mg/g (Table 1).
3.1.3 HPLC profile
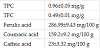
Reverse-phase HPLC system was used for identification and quantification of phenolic compounds present in supina grass extracts (Table 1 and Figure 1). The most abundant phenols were caffeic acid (23 ± 3.32 mg/ 100 g), coumaric acid (159.2 ± 9.2 mg/100 g) and ferulic acid (286.99 ± 9.43 mg/100 g). In another study, ferulic acid was the most predominant phenolic in supina grass extracts, which supports our results [44]. However, ferulic acid quantities were lower in the latter study (30.9 ± 1.99 mg/100 g), which may be due to the extraction method was used as only 1 set of parameters with a single solvent system was applied to the grass.
3.2 Effect of Supina grass extracts to remediate C. albicans (SC5314 and A72) biofilm formation
In presence work, the biofilm formation effect of a supina grass extract was determined at four-time points (1, 3, 6 and 24 h) using five different concentrations (0.72, 7.2, 72, 720, and 7200 ng/g). The effectiveness of the extracts were analyzed by statistically comparing the four time points that showed remediation at a given concentration (Figures 2 and 3) for two strains of C. albicans, A72 and SC5314.
3.2.1 Remediating C. albicans (A72) biofilm formation
The extracts were analyzed further by comparing the four time points that showed remediation at a given concentration (Figures 2) for C. albicans (A72 and SC5314). For C. albicans (A72), after incubation with the supina grass extracts, only the 24 h time point resulted in different responses among concentrations as % biofilm remediation of biofilm ranged from a low -0.29 % (7.2 ng/g) to high of 11.93 % (0.72 ng/g). As the concentration increased to (7.2 ng/g), the 24 h started to trend differently resulting in a low of ~ -0 % (24 h) to a high of 25.33 % (1 h), 30.51% (6 h) (Figure 3).
Moreover, at a (72 ng/g) dosage, the responses ranged from a low of 14.85% (24 h) to a high of 44.46% (3 h), and the 1 and 3 h trend were different at the same concentration, i.e., with a % remediation of >40 % (3 h) and ~ 17 % (1 h). Again, it is clear that the 1 and 24 h time responses were not as efficient on remediating biofilm formation by C. albicans (A72).
These results indicated that supina grass extracts can potentially act as efficient antifungal agent. The extracts were at the different concentrations able to remediate biofilm remediation more effectively at 1 and 24 h. This remediation action is strongly attributed to the cinnamic derivatives (CA, F, and C), which are commonly present with other polyphenolic compounds with higher antioxidant capacity [42]. As proposed by Borges et al. [47] the compounds that have exert high antioxidant capacity to scavenge free radicals also represent high antimicrobial activity [47], as microbes harm effects are cause in part by their ability to release abundant reactive oxidative species.
3.2.2 Remediating C.albicans (SC5314) biofilm
In the case of (SC5314), the %remediation was significantly different among time points. At 24 h exposure treatments were not similar relative to 1 and 3 h, which in turn were statistically different from one another (Figure 3). Indeed, the 24 h exposure time at the low concentration (0.72 ng/g) exhibit a % remediation of a low of ~4%, while the effect was higher for 1 and 3 h (24.43 and 45.5 %). When the concentration increased to (7.2 ng/g), the % remediation was 27.15 % (24 h), which was statistically different from 47.82 % (3 h). At 72 ng/g, the 1, 3, 6, and 24 h elicited a biofilm reduction of 7.58 % (1h), 36.29 % (3 h), 30.81 % (6 h) and 18.89 % (24 h), with the 3 and 6 h were statistically similar to one another. At (720 ng/g), the (3 h) caused a high reduction (34.32 %), while the % decreased to a low of 19.75 % (24 h). Also, the (1 h) incubation time was statistically differently from 3 and 6 h (720 ng/g), and from 24 h (7200 ng/g). It should be noted that the (3 and 6 h) were the most effective time to reduce established biofilms produced by the SC5314 C. abicans strain among concentrations. Accordingly, (1 h) is not enough time to allow penetration into cells to alter biofilm formation or to act directly upon it structure, while after (24 h) extracts also started to aid the biofilm formation as % remediation declined relative to the (3, and 6 h) time points.
Supina grass extracts have shown a potential on remediation C. albicans (SC5314 and A72) biofilm formation via different means. Indeed, For biofilm formation, the 24 and 1 h were the least effective particularly at low concentrations for both strains. As it was suggested by Cerca et al. [48] that the inhibition of cellular adherence by one approach necessary contributes to biofilm formation inhibition because they are two district phenomena.
Usage of plants as a traditional medicine that can potentially prevent and treat diseases has been a common practice in antiquity but has increased in intensity during the modern age [49,50,42]. In terms of C. albicans infections, Because of its cost-efficiency and a rich discovery herbal approaches is an ideal strategy to prevent cell pathogenicity, and thus control biofilm formation due to the biodiversity of a plant, cost-efficiency and sustainability. Indeed, studies completed in our laboratory showed that phenols are more potent in combination (data not shown) than the sum of the individual components (data not shown). Therefore, supina grass may promising under-utilized agro-product for reducing already established C. albicans cells to several surfaces [51] and thus offer a potentially novel alternative to currently used anit-adhesive approaches while averting resistance to such treatments [52].
As it was confirmed by HPLC analysis shown in (Figure 1), supina grass extracts contained high quantities of phenolic compounds including coumaric (C), ferulic (F), and carboxylic acid, which are hydroxylated derivatives of cinnamic acid, but the hydroxyl groups numbers and position on the aromatic ring and substitutes type results in potential differences on phenolics characteristics [47].
Phenolic antifungal extracts target the fungal membrane and its components as a common mechanism of action [52]. Jothy et al. [53] investigated the anticandidal mechanism of action of methanol extract of Cassia fistula Linn. The extracts entered and disrupted the plasma membrane. The extracts accumulated in the plasma membrane, which caused mitigation of cell growth. Several studies support that F, CA, and C effectively mitigated fungal infections by disrupting the cell cytoplasmic membrane [54-56].
3.3 Synergistic effects of phenolic compounds with supina extracts combined with phenols on remediating C. albicans (A72 and SC5314)
In this study, supina grass extracts was combined with ferulic (F), and coumaric (C) acid because they are the main phenolics identified in this system. Five concentrations of supina extract (0.72, 7.2, 72, 720, and 7200 ng/g) were combined with four concentrations of F and C (0.03, 0.06, 0.13, and 0.25 mM) that showed effectiveness in reducing biofilm formation either as isolated components or when combined with another phenol. The (6 h) response time was studied because it shows the maximum remediation effect in most of the cases. Herbal efficacy is often debated to be due to the chemically diverse components present in the natural system, which are distinct in concentrations and constituents. Thus, they may act synergistically or additively to contribute such a positive effect [57]. The literature has demonstrated that mixtures of phytochemical have a high capacity of acting as synergists rather than an isolate [58,20].
3.3.1 Remediating biofilm formation of C. albicans (A72)
C. albicans A72 demonstrated similar remediation effects among low compounds supina extract- C and supina extract - F extract at concentrations tested (Figure 4), which ranged from (~ 20 % to < 40 %) supina grass extract- C, while supina extract - F ranged from (12% to > 50%). However, the biofilm remediation effect trended differently at some of the concentrations as (0.72 and 7.2 ng/g /0.03 mM) exhibited ~ 19 % (supina extract- C), > 50 % (supina extract - F) >20 (supina extract-F), and ~ 45 % (supina extract - F), respectively. The addition of (0.06 mM) C and F to (7.2 and 7200 ng/g SE) showed a low of (~ 23 to a high of 37.64 %) when treated with supina grass extract- C and a low of (~ 9 % to a high of 21.45 %) supina grass extracts when exposed to extract-F remediation effect, respectively. As the concentrations of supina grass extract- C and supina extract- F increased to (7200 ng/g/ 0.13 mM), the remediation effect was significantly different as reduction was respectively (37.04 %).
In a study preformed on demonstrating the antimicrobial activity of olive mill wastewater (OMW) in combination with phenolic compounds including ascorbic acid, tyrosol, protocatechuic acid, vanillic acid, caffeic acid, gallic acid, ferulic acid, and p-coumaric acid. The results indicated that these combinations demonstrated complete reduction of gram-positive (streptococcus pyogenes and staphylococcus aureus) and Gram-negative bacteria (escherichia coli and klebsiella pneumoniae), also, the extracts and phenolic compounds had synergic effect rather than when tested alone [59]. In support of this data, a study completed by P. major extract and a combination of its two major compounds (aucubin, and baicalein) demonstrated strong alleviation of C. albicans biofilm formation in dose dependent manner [60].
3.3.2 Remediating biofilm formation of C. albicans (SC5314)
The remediation potential of supina extract - C and supina extract - F were statistically different in the case of C. albicans SC5314 (P< 0.05). Supina extract - C showed high percent remediation that ranged from (~ 29 % to > 50 %) at the concentrations tested (Figure 5). On the other hand, supina extract - F exhibited low percent biofilm remediation as ranged from a low of (~ 9 % to a high of ~ 26 %). These results again demonstrated the high efficacy induced by the addition of C to supina extract comparison to F. The SEC and supina extract - F were less effective on mitigating C. albicans (A72 and SC5314) biofilm formation (> 70 %), which may be attributed to the complexity of biofilm [60].
4. Conclusions
Supina grass extracts were the most effective on remediating the two strains of C. albicans (SC5314 and A72) biofilm formation at an incubation time 3 and 6 h time response. However, upon combining the extracts with C or extracts with F, the remediation effect of biofilm formation was higher > 50 % comparison to biofilm formation < 50 %, which indicates that the phenols and extracts were acting synergistically, as also supported by the FIC <0.5. Therefore, the significance of this study is that a sustainable co-product stream could be used as a source of anti-fungal agents with phenol synergists most likely playing an important role. Moreover, as complex matrix, supina grass grass enriched phenols or phenols extracted from the this natural system may be more effective in targeting C. albicans virulence phenotypes and also acting on multi-targets thereby aiding in preventing resistance to a potentially novel anti-fungal agent.
Competing Interests
The authors declare that they have no competing interests. The author declare that there is no competing interests regarding the publication of this article.