1. Introduction
Mild Cognitive Impairment (MCI) is considered a stage between normal cognitive ageing and dementia characterized by cognitive impairment, preserved activities of daily living and either intact or minimally impaired complex instrumental functions [1]. Approximately 10 percent of cases, yearly, advance to Alzheimer's disease (AD) [2]. The numbers vary in different studies and the patients are often followed for many years as no markers exist today that with certainty distinguishes patients that in the future will convert from MCI to a dementia disease. The term MCI has different definitions but one of the most commonly used has been to describe MCI as memory impairment beyond normal cognitive aging.
As no effective curative therapy is yet available, it is of greatest importance to reduce risks for developing cognitive diseases through interventions and mapping of risk factors. An increased knowledge and awareness of influential life style factors and pathogenic factors such as diabetes, midlife obesity, depression, physical and mental inactivity, smoking, midlife hypertension, level of education and diet has led to treatment strategies for both primary and secondary preventive purposes [3-6]. The relation between physical activity, metabolic factors, inflammatory markers, mild cognitive disease and dementia has been studied both with the purpose of early diagnosis and to predict conversion from MCI to AD [7,8].
Pathophysiological alteration of inflammatory markers, such as cytokines, has been studied, and elevated levels of tumor necrosis factor alfa (TNF-α), interleukine (IL) IL-6, IL-8, IL-10, cyklooxygenas 2 (COX-2) and interferon alfa (INFα) has been found in MCI patients compared to controls [8,9]. Also, elevated levels of the cytokines IL-6, IL-1β and TNF-α [10-14] has been found in depressed patients which is of interest since depression is common in patients diagnosed with MCI. Additionally, inflammatory changes are induced when fatty tissues enlarge (obesity) which can be seen in an increased production of cytokines, then named adipokines (TNF-α and monocyte chemotactic protein 1 (MCP1)) [15,16]. Moreover, inactivity has been associated with inflammation (having increased IL-6 and C-reactive protein (CRP)) independently of obesity, gender, age and smoking [17].
Physical activity and training has a lot of known beneficial physiological effects, for instance, on the circulatory system; with increased stroke volume, increased capillarization, decreased blood pressure and on the metabolism; with increased mitochondrial function leading to increased aerobic capacity, decreased blood glucose levels and decreased insulin resistance, decreased blood lipids with increase of HDL [16,18,19]. Thus, training counteracts life styleinduced factors such as diabetes, obesity and hypertension [20,21] (in 20 chapter 26, 36, 31). Moreover, moderate training as well as a single training bout provides general improvement in the immune system [22]. Also, training has been shown to have anti-depressive effects [20,23](in 18, chapter 24). In older people improved memory and increased hippocampal volume has been observed after aerobic training [24] and exercise during middle age has been shown to give positive long-term improving effects on cognitive functions and protect against onset of dementia [25,26]. In patients with MCI aerobic training and/or strength training has also been shown to improve cognition, global cognitive ability and memory [27-29].
Also, biochemical improvements of brain derived neurotrophic factor (BDNF), cholesterol, testosterone, estradiol, dehydroepiandrosterone, and insulin has been observed after training in patients with MCI or dementia [30]. The dose of training to obtain the best outcome has been in focus, where high intensity training improvesmaximal oxygen uptake (VO2max)in a greater extent, compared to endurance training1,2 at lower intensities, in younger persons [31].
During skeletal muscle contractions different cytokines, in this context named myokines are released [32-34]. The myokines have been shown to have autocrine, paracrine and endocrine effects. As examples, in the working muscles glucose and fat-uptake are increased (IL-6, BDNF), the growth of capillaries nearby are stimulated (IL- 8, CXCL-1?), glucose-releases from the liver (IL-6) and fat-releases from the adipose tissue is stimulated (IL-6, Irisin) [35-38]. Also, antiinflammatory effects have been shown, whereIL-6 from the muscles has a direct anti-inflammatory effect due to inhibition of TNF-α and an indirect effect due to release of anti-inflammatory 1L-1ra and IL- 10, which antagonize the pro-inflammatory TNF-α, IL-1β and IL-8 [15,25,39-41].
The release of myokines has been shown directly as a response to single exercise bouts< 3 h,and the ability to generate and secrete myokines has been shown to increase after aerobic and strength training> 8 weeks [42]. Our assumption is that a single exercise test of 10-12 min duration with increased inclination to maximal workload and corresponding increase in muscular work, could be a possible and effective way to study the stimulated release of myokines.
Apart from this possibility, the exercise test combined with ECGor pulse measurement and ergospriometry, is an excellent test to perform, giving the measured maximal heart rate and oxygen uptake, which are useful when prescribing physical activities with individualized intensity. The exercise test is also a gold standard for determination of the aerobic capacity (peak oxygen uptake, VO2peak), which is useful in order to follow the effect of training.
Our hypothesis was that a blood sample taken during a rest period would show levels of cytokines that mirror inflammatory processes. We assumed that myokine levels would increase during muscle work at the single exercise test and that the effects from interventional training lower the levels of inflammatory cytokines at rest (i.e.IL-6, IL-1β, IL-8 and TNF-α) and that the anti-inflammatory myokine levels produced by muscle work (i.e.IL-6) are increased after a training period. Our assumption also was that high intensity training gains more effects than moderate intensity.
The present study was intended as a pilot study to explore if it is possible to detect changes in cytokine/myokine levels in response to a single exercise test, with measurements at rest and at maximal workload, in patients with MCI. Further the aim was to explore if the levels of cytokine/myokine changes after a period of aerobic exercise and strength endurance trainingwith two different intensities.
2. Participants and Method
2.1 Participants
The recruitment process started with letters asking for interest to participate in the study to 234 patients with mild cognitive impairment (MCI) diagnosed at two Memory Clinics (135 and 99 patents respectively) who had visited the clinics in a 2-year time span. Seventy persons showed interest and their medical journals were checked with respect to inclusion and exclusion criteria whereafter 20 persons were excluded and additional 4 persons declined participation.
The inclusion criteria were; 60-80 year, a Mini Mental State Examination ≥ 24, and a medical investigation the latter 12 months or consecutive new patients. The exclusion criteria were; dementia, other known brain disease or brain injury explaining the cognitive decline, serious psychological disturbance, medical obstacles that counteract exercise tests or training or deficient knowledge of Swedish language.
Thus 46 persons were invited to participate of which 42 accepted to be included in the project and were randomized into the three groups. Six persons declined participation before the initial test session, 3 due to transport difficulties and 3 due to joint problems (back, knee, and foot).
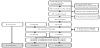
Finally 36 participants were included into a control group (CG) (n=8), a moderate intensity training group (MITG) (n=13) and a high intensity training group (HITG) (n=15). One subject in the moderate intensity group performed the initial tests but did not show up to the exercise sessions due to a longer vacation abroad. Two subject in the high intensity group stopped exercise, one after 2 sessions due to hip arthrosis and one after 3 sessions due to extreme tiredness. Thus 25 participants fulfilled exercise training period and the baseline and post training-tests. Analyses of the actual intensities during the 12 weeks of training were performed using the recorded heart rate (HR), which showed that 3 participants in the moderate group had performed exercise with higher heart rate than prescribed. Additional 4 participants in the high intensity group had an actual exercise intensity lower than prescribed. Thus a shift of these 7 participants were made and analyses performed based on actually exercise intensities were made having 13 participants in the MITG and 12 in the HITG, see figure 1.
2.2 Exercise test
A maximal incremental exercise test was performed on an ergometer cycle (Crescent 939E, Monark, Cycleurupe®, Sweden). Measurements of oxygen and carbon dioxide (ergospirometry) were performed using Oxycon pro™ (Carefusion, Hoechberg, Germany) with breath-by-breath sampling and registration every 10th second. The test protocols were designed with an initial load of 30W and an increase of 5W/30s for women and an initial load of 50W followed by an increase of 5W/20s for men according to Astrom [43]. ECG was measured at rest and continuously during exercise. Blood pressure and rating of perceived exertion (RPE) on Borg 6-20 RPE scale [44] were performed every second minute. The test was ended when the subject rated 19-20 on the RPE scale, or if any clinical objective were detected, or any subjective symptoms were reported. Maximal values of RPE and heart rate (HR) were registered in order to prescribe exercise intensity. Maximal heart rate (beats/min) was defined as the mean of six 10s registration at maximal workload. Experienced exercise physiologist/physiotherapist and an experienced cardiologist were in charge for the tests. A defibrillator was available in case of need and the personal master the heart-lung rescue procedure.
2.3 Blood samples
Venous blood samples were collected from v. brachialis prior (at rest) and directly after the maximal exercise test (maximal workload), stored in refrigerator and transported to the Immunological Laboratory, Skånes University Hospital, Lund for analyse of the cytokines/ myolines TNF-α, IL-6, IL-1β and IL-8.
2.4 Analyses of cytokines/myokines
The method used to detect cytokines in plasma and serum is a commercial Quantikine HS ELISA Kit from R&D-systems, Inc. (Minneapolis, USA), for details see links [45]. IL-6, IL-1β and TNF-α was analysed in serum and IL-8 in plasma. The following reference values were applied for estimating inflammation; IL-6<8ng/L, IL1B <5 ng/L, TNF <15ng/L and IL-8<60ng/L[46].
2.5 Training intervention
Training was performed one hour two times a week for 12 weeks in two groups, one with moderate intensity training (MITG) and one with high intensity training (HITG) according to Northons intensity classification [47].
Each individual was given a range of HR as well as a range of perceived exertion, based on the initial maximal exercise test, as an exercise recommendation for guidance during the training. Those in the high intensity group were given a range of HR of 70-90% HRmax with the corresponding perceived exertion varying between 7-17 on the RPE-scale and those in the moderate intensity group were given a range of HR of 55-70% HRmax with the corresponding perceived exertion varying between 6-13 on the RPE-scale.When prescribing exercise using a range of heart rate (based on a previous exercise test) – an assumption is that during the training period the participant has to adjust/increase the load (or power) because a training effect is that the sub-maximal heart rate becomes lower.
An experienced physical leader supervised the exercise. Aerobic training was performed on stationary bicycles and synchro-machines, using interval training. Endurance strength training was performed using different weight training machines. Each session had the same construction, starting with a warming up standing freely in the room during 6 min, followed by 5 min aerobic on bicycle or synchromachines, four different exercises of 2 min strength endurance, 10 min aerobic on bicycle or synchro-machines, three different exercises of 2 min strength endurance, 10 min aerobics on bicycle or synchromachines, 4 min of cooling down and additional 4 min of stretching standing freely in the room.
During each training session HR was monitored by using Polar 300 chest belt placed around thorax connected to a wrist unit (Polar Electro Oy, Kempere, Finland). Each individual used the same wrist unit during all exercise sessions. Each participant had their own diary with clear instruction of intensity and after each exercise part they were asked to manually write down the HR as well as their perceived exertion. After the training period all the registrations of HR from the Polar equipment were transferred to a computer and analysed in order to ensure that the exercise intensities performed were in accordance with the prescribed intensity. These analyses resulted to the shift of participants between groups described above, with the motivation that actual performed intensity during exercise are physiological correct to analyse than prescribed not fulfilled intensities.
The participants in the control group (CG) were asked to continue with their ordinary activities and were not given any training regimes.
2.6 Test and training procedure
Blood samples were collected at rest and directly after the maximal exercise test at baseline and also after the 12 weeks intervention. The test- and training procedure is shown in figure 2.
2.7 Statistics
The characteristics of the participants are presented as number, mean (min-max) or year span. Cytokine/myokine levels are shown as median (min-max) and the differences are presented as median (25th-75th percentile) and as median%. To assess difference Wilcoxon Signed Rank test was used within groups and Kruskal-Wallis test was used between groups. The statistical tests were performed in IBM SPSS Statistics 22 for Windows (IBM Corporation, Armonk, NY, U.S.). A significance level of 0.05 was chosen.
2.8 Ethical approval
The study was approved by the Regional Ethical Review Board in Lund, Sweden (2014/6). Each participant signed a written informed consent after written and oral information.
3. Results
The characteristics of the 33 participants in this study are shown in Table 1. Twenty-five participants completed the training period and the baseline- and post- exercise tests (13 in the moderate intensity training group MITG, 12 in the high intensity training group HITG), and 8 participants completed the baseline- and post-exercise tests as controls, (CG). Male gender was more prevalent in all three groups. There were no differences in age, body weight, body height or BMI between the three groups. Fourteen participants were classified as overweight or obese according to the WHO classification system [48]. Also, 17 of the participants subjectively reported problems with balance and/or vertigo (4 in CG, 6 in MITG and 7 in HITG).
Resting values in all participants of the cytokines TNF-α,IL-6, and IL -8 were normal compared to the reference values (see method section). Normal resting values were also seen for IL-1β in all participants except one in MITG at retest and one in HITG at baseline. This indicates that no inflammatory process were active in this group of MCI patients, neither at baseline nor after the 12 weeks intervention.
The baseline measurements at rest and directly after the maximal exercise test (maximal workload) of cytokines/myokines for all participants are shown in Table 2. It can be seen that the median values for all four cytokines/myokines are higher after the maximal exercise test than at rest, with a significantly increased level of IL-6 (p= 0.002), TNF-α (p<0.001) and IL-8 (p=0.020).
The baseline measurements at rest and directly after the maximal exercise test (maximal workload) of cytokines/myokines for the participants spit into training groups and controls are presented in Table 2. It can be seen that the median values forIL-1β in MITG and IL-8 in HITG was lower at maximal workload than at rest. Otherwise, the same patterns with higher values of cytokines/myokines after the maximal exercise test compared to at rest can be observed in the three groups similar to all participants baseline values, but with significant increased levels only for TNF-α (p=0.016) in the CG and for IL-6 (p= 0.001) and IL-8 (p= 0.012) in the MITG.
During the 12 weeks of training 24 sessions were possible to attend, the mean (min-max) session attended by the MITG was 21 (16-24) and by the HITG 21 (17-24). During the training period one person reported influenza infection, but still took part in 22 training sessions.
After the 12 weeks of training, a maximal exercise test with measurements of the cytokines/myokines was performed again. The retest measurements at rest and directly after the maximal exercise test (maximal workload) of cytokines/myokines for the training groups and controls are presented in Table 2.It can be seen that the values for TNF-α and IL-8 in HITG was lower at maximal workload than at rest. Otherwise, the same patterns with higher values of cytokines/ myokines after the maximal exercise test compared to at rest were observed in the three groups as at baseline, now with significantly increased levels for IL-6 and for IL-1β in MITG (p= 0.002 and p=0.011 respectively) and in HITG (p=0.003 andp=0.037 respectively).No significant differences of the cytokine/myokine levels at rest compared with maximal workload were detected in the CG, Table 2.
Within group comparison of cytokine/myokine levels at rest (retest-baseline) showed significantly lower values at retest for IL-6 (P=0.05) in the HITG. No other significant within group differences of resting values were shown in CG, MITG or HITG.
Within group comparison of IL-6, IL-1β, TNF-α, IL-8 levels at maximal load (retest-baseline) showed no significant changes in CG, MITG or HITG.
Comparisons of the differences of the cytokine/myokine levels at single exercise test (max-rest) between the groups using Kruskal- Wallis test were non-significant, both at baseline and at the retest.
Also, baseline and retest comparisons of the differences of the cytokine/myokine levels of resting values (rest at retest- rest at baseline) and of maximal workload (maximal workload at retest-maximal workload at baseline) between the groups using Kruskal- Wallis test were non-significant.
4. Discussion
4.1 Main findings
The main findings of our study are;
- Normal resting values of the cytokines indicates that no inflammatory processesare activein this group of MCI patients.
- A single exercise test significantly elevated the levels of myokines IL-6, TNF-α and IL-8 in the MCI group. The results were not identically reflected when the MCI group were divided into intervention groups, probably due to the small sample size in each of the intervention groups.
- After a training period of 12 weeks a reduction of the cytokine IL-6 was seen at rest compared with baseline in the high intensity group.
- After a training period an increased production of myokine-IL-6 and IL-1β were seen in the MITG and in the HITG in response to a single exercise test. In addition, the levels of TNF-α and IL-8 in HITG were lower at maximal workload than at rest.
- The comparisons between the groups were not statistically significant.
These findings will be further discussed below.
4.2 Participants
The recruitment of participants to the study started with letters asking for their interest to participate. Letters were sent to a total of 234 patients with MCI, and 70 patients were positive to participate. Finally 36 of these took part in the study. We assume that our participants were highly motivated to training and maybe also initially more fit than the large cohort, thus not fully representative for MCI patients. However, some of the pathogenic factors mentioned in the introduction [3-6] werepredominant in our sample. Nearly half of the participants were overweighed or slightly obese, as indicated by the medications; one third were treated for hypertension and also one third were treated for circulatory problems (anti-thrombosis treatment). But only a smaller part of the participants were treated for depression (4 participants), diabetes (2 participants) or inflammation (2 participants). Thus, comorbidity existed in the group studied. Furthermore, the participants were heterogeneous regarding the duration of the MCI condition indicating different aetiologies and hence different expected course of events both with and without exercise.
4.3 Cytokines
Normal resting values of the cytokines, both at baseline and after the training period, indicates lack of an inflammatory process in this group of MCI participants. This is in accordance with a previous review by Saalem [49], where no significant differences in inflammatory factors were seen between MCI and controls, although some heterogeneity were observed.
This means that our hypothesis that ourgroup of MCI patients would show levels of cytokines that mirror inflammatory processes were not supported.
Among the pathophysiological mechanisms discussed in depression an inflammatory subtype has been defined, including inflammation related processes like platelet activation, oxidative stress and damage to mitochondria [11]. Analogously, we speculate that there could be a subtype of MCI with inflammation, or as suggested by other studies, the inflammatory process might be a possible mechanism in the conversion of MCI to AD [7,8].
Thus, despite our findings of normal resting values of cytokines, continuous measurement of inflammatory markers are of interest, to further explore if there is a subtype with inflammatory processes in MCI patients and also in clinical long-term follow up to detect conversion to AD.
4.4 Single exercise test and myokine production
The single exercise test with incremental increase allows the person tested to reach maximal workload in 10-15 minutes and involves muscular work for large muscle groups in the legs. The results from this study showed significantly increased levels from rest to maximal load of the myokines IL-6, TNF-α and IL-8 in the MCI group (N=33), also a non-significant increased level of IL-1β was seen. When splitting the sample of all participants into the intervention groups increased levels from rest to maximal load of all myokines were observed, but only significant for TNF-α in CG, and for IL-6 and IL-8 in MITG, probably due to the small sample size.
As shown in this study with older persons with MCI and also in a previous study with younger depressed persons [50], the muscle work performed in a single exercise test is enough to increase the myokines IL-6, IL-8, TNF-α. Thus, the single exercise test with blood-samples taken at maximal workload is a timesaving possibility to explore the release of myokines compared to longer single exercise bout with blood-samples previously described [42].
Thus, our hypothesis that myokine levels would increase during muscle work at a single exercise test in persons with MCI could be accepted. In this study, we have not included age-matched healthy controls, thus we cannot tell if the response would be the same. This is of particular interest to study since age-related differences have been observed between healthy young and middle-aged women in some cytokines 1 hour after a single bout of high intensity training [51].
4.5 Training
The adherence to the training sessions was remarkably high (average 21 of 24 sessions) in both exercise groups. The prescription of exercise intensity was based on each persons maximal exercise test and given in a range of training heart rate of 55-70% HRmax or 70- 90% HRmax, corresponding either to moderate or high intensity. In practise this means that during the training period the participant increased their power on the stationary bicycles and syncro-machines and the load at the endurance strength machines in order to withhold the prescribed rage of heart rate. However, the exercise intensity prescribed was difficult to follow for 7 participants (as described in the method section), which was detected when we analysed the HR data collected during the 12 weeks. Three participants in the MITG found the training inspiring and joyful and questioned the, in their experienced, low prescribed intensity, and thus chose to increase their intensity. While 4 participants in the HITG found the intensity prescribed to high and then decreased their intensity. When analysing the effect of the training we therefore switched groups for these 7 participants. For further studies our experience is useful, leading to the recommendation to check actually performed exercise intensities (not only prescribed intensities) in order to ascertain the dose of training each participant has completed.
4.6 Training effect on cytokine levels
After the 12-weeks training,a significant reduction of the cytokine IL 6 was seen at rest at the re-test compared with rest at the baseline test in the high intensity group.This might indicate a traininginduced reduction of inflammatory response. As described above and in the results, the participants in this study showed normal resting values both at baseline and after the training period, indicating no inflammatory processes. Our hypothesis that both moderate and high intensity training would lower the levels of IL-6, IL-1β and TNF-α has to be mainly rejected. Other studies have shown reduced inflammatory markers after training [40], in particular studies with participants with elevated inflammatory markers to start with. Thus in order to justify our hypothesis and show reductions of inflammatory cytokines, this would need to be studied with participants having inflammatory cytokines elevated at baseline.
4.7 Moderate intensity training effects on myokine levels
After the 12-weeks training, a significant increase in the myokine-production of IL-6 from rest to maximal workload was seen at the single exercise test in MITG. This is beneficial according to the proposed effect of IL-6 to antagonize the pro-inflammatory IL- 1β, IL-8 and TNF-α [40,41]. However, no reduction of the pro-inflammatory myokines was seen at maximal load in the single exercise test performed in the MITG. Instead the level of exercise induced pro-inflammatory IL-1β was increased from rest to maximal load andTNF-α and IL-8 showed no significant changes from rest to maximal load. Thus, no training-induced reduction of exercise-induced pro-inflammatory myokines was seen in the MITG.
4.8 High intensity training effects on myokine levels
After training a significant increase in the anti-inflammatory myokine-production of IL-6 from rest to maximal workload was seen at the single exercise test in HITG. As in the MITG, also the pro-inflammatory myokine IL-1β increased from rest to maximal load, but a reduction of the exercise-induced pro-inflammatory TNF-α and IL8 were seen which might indicate a training-induced lower inflammation response in HITG.
The within group comparisons above provide support for the hypothesis that training has beneficial effects stimulating antiinflammatory production of IL-6, and it seems that high intensity training lower the levels of some of the pro-inflammatory myokines (TNF-α and IL8), which also was in accordance with our hypothesis.
However, no differences between the groups were seen when we compared the controls and the two training groups, a result we assume is due to the small sample size in this pilot study. Also, it would be of interest to study the exercise-induced myokine-production in participants having inflammatory cytokines elevated at baseline.
5. Conclusion
Our results indicate that the method to explore cytokines/myokines using a single exercise test could be a useful complement to simply analyse resting values and that positive protective effects of training thus might be detectable.
In the group of MCI participants a training period of 12 weeks with high intensity altered the cytokine/myokine response, which might indicate a training-induced reduction of inflammatory response.
For further studies larger patient groups and also longer period of training intervention may be desirable in order to increase the knowledge of the myokine release and its possible preventive effects on cognitive impairment.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Design of the study: AW. SV. Analyzed the data: HJ, AW, MEBL Wrote the first draft of the manuscript: MEBL Continued writing the manuscript: AW Contributed to the writing of the manuscript: HJ, MEBL, SV, LW Agree with manuscript results and conclusions: MEBL, HJ, LW, SV, AW Jointly developed the structure and arguments for the paper: MEBL, AW Made critical revisions and approved final version: MEBL, HJ, LW, SV, AW All authors reviewed and approved of the final manuscript.
Acknowledgments
We are grateful to all patients who participated in tests and training. Special thanks, to Åsa Tornberg, Stephen Garland, Stig Persson, Adela Briznik, Gunilla Gidö and Lisa Bjartmar, for their professional contributions with data collection and training.