1. Introduction
Kawasaki disease (KD), a condition that occurs most frequently in infants, is characterized by systemic vasculitis of the small and medium arteries [1]. The underlying mechanisms by which KD triggers vasculitis remain unclear, despite considerable research. However, certain stimuli that generate and release various inflammatory substances within the body, principally infections, are known to trigger vasculitis, causing panangiitis through complex interactions.
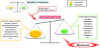
When exposed to inflammatory substances, vascular endothelial cells partially contract, forming intercellular gaps. Plasma proteins and other substances that are normally contained within the blood vessels exude from these gaps, forming chemotactic factors. Monocytes and macrophages then infiltrate the site of inflammation, become activated, and produce reactive oxygen species (ROS) [2].
The human body has evolved a system for maintaining redox homeostasis in response to various external stresses. This system begins to scavenge ROS to counteract the production of ROS by the activated immune cells. A healthy individual maintains a proper balance between their ROS generating and scavenging systems. If, however, the balance tilts toward the production of ROS, the excess ROS accumulate, leading to oxidative stress [3,4] (Figure 1). Excess ROS attack proteins, carbohydrates, lipids, nucleic acids, and other constituent substances of the body, resulting in cellular damage that is believed to cause a variety of diseases. In KD specifically, the excess ROS produced by infiltrating inflammatory cells create cytotoxicity that is presumed to play a role in the pathogenesis of vasculitis.
2. Phlogogenous Substances Involved in the Formation of Acute Vasculitis
It is no exaggeration to state that ROS are involved in all proinflammatory pathways. Infiltrating neutrophils and macrophages are the primary sources of the ROS produced during inflammation. Activation of NADH/NADPH oxidase in these inflammatory cells causes rapid and abundant production of ROS that can lead to a state of oxidative stress if not removed by the ROS scavenging system. The arachidonic acid cascade, the activation of xanthine oxidase in vascular endothelial cells [5], or the inhibition of the electron transport system in intracellular mitochondria can also cause overproduction of ROS.
ROS are thought to affect cell function via two mechanisms. The first mechanism involves cellular impairment that occurs through the direct interaction of cellular components with ROS. In the second mechanism, ROS act as second messengers that induce the production of other inflammatory substances, such as tumour necrosis factor (TNF)-α [6-8].
Neutrophil elastase (NE), a substance released from activated neutrophils, is also an important player in the acute inflammatory response [9]. NE is a powerful proteolytic enzyme with low substrate specificity. Infiltration of coronary artery lesions by NE-positive neutrophils has been proven histologically by immunostaining.
The neutrophils, macrophages, and vascular smooth muscle cells that accumulate at the site of inflammation are also known to transiently produce large amounts of nitric oxide (NO) through the action of inducible NO synthase (iNOS) [10]. NO is an unstable radical that forms peroxynitrite (ONOO-) in the presence of excess ROS. NO initially acts as an endothelium-derived relaxing factor (EDRF). Vessels that become resistant to NO constrict when their sensitivity to NO is lost, exacerbating the effects of inflammation on the dynamics of blood circulation. In addition, ONOO- creates highly reactive radicals that cause significant tissue damage and are thought to induce edema via promotion of vascular permeability.
In summary, the production of various inflammatory substances, including ROS, is positively correlated and highly damaging to tissue. These substances are thought to be involved in the etiology of the acute vasculitis associated with KD.
3. Dynamics of Oxidative Stress in the Acute Phase of Kawasaki Disease
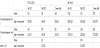
We measured the blood reactive oxygen metabolites (ROM) level, which is an index of the activity of the ROS generating system, as well as the serum biological antioxidant potential (BAP), which is an index of the ROS scavenging system, in patients with acute KD [11] (Table 1).
The subjects included 19 patients with acute KD (Table 2). Thirteen of these patients responded favourably to the first course of intravenous immunoglobulin (IVIG) treatment (2 g/kg in 1 dose). The remaining 6 patients did not respond favorably to the first course of IVIG. In all cases, blood ROM and BAP levels were measured immediately prior to IVIG treatment, immediately after IVIG treatment (24 h after the end of administration), and 2 weeks after the end of IVIG treatment (Figure 2). In the group of patients who responded poorly to the first round of IVIG, treatment with either additional IVIG or steroids resulted in resolution of fever within 1 week. Although the blood ROM levels were clearly elevated immediately prior to IVIG in the group that responded favorably to treatment, the initial course of IVIG caused a favorable decline. In the group that responded poorly, however, no decline was observed after the initial course of IVIG treatment, even though their ROM levels immediately before IVIG were similar to those in the group that responded favorably. However, there was a significant drop after 2 weeks. No clear fluctuation in BAP levels was observed before or after IVIG treatment in the group of patients who responded favorably to IVIG, but levels tended to gradually increase 2 weeks after IVIG treatment. In the group of patients who responded poorly, however, no significant change in BAP was noted throughout the entire course. The BAP levels immediately before IVIG treatment were significantly lower in the group of patients who responded poorly than in the group that responded favorably (p < 0.01).
These results suggest that ROS generation is significantly enhanced in acute KD, and drops immediately with IVIG treatment. This finding, when used in concert with a reduction of inflammation observed via an independent mechanism, may be useful for determining therapeutic efficacy. In addition, the slight lag in the activation of the ROS scavenging system behind that of the ROS generating system suggests that the ROS scavenging system has a gradual functional increase triggered by the stimulus of increased ROS. However, it is also possible that the capacity of the scavenging system might regulate responsiveness to IVIG treatment and the subsequent ability to recover from the damage sustained during the acute phase.
4. Future Prospects for Acute-phase Treatments
Current acute-phase treatments focus on reducing inflammation, and there is a consensus that IVIG is the first line of defense [12]. The results of the recent large-scale RAISE STUDY trial have also brought the efficacy of steroid administration under review [13].
The growing diversity of acute-phase treatments is illustrated by the increasing numbers of clinical reports on the effectiveness of anti- TNF-α antibodies, which are not currently indicated for KD [14,15].
Coronary artery involvement is regarded as the most serious complication in acute KD. While the degree of coronary involvement has followed a downward trend in association with the growing diversity of treatments, the problem has not yet been eliminated. The significant role of oxidative stress in the etiology of acute vasculitis, which our research documents, is an important consideration in the development of new treatment strategies. Control of oxidative stress, a factor closely related with inflammation, may quickly alleviate acute inflammation.
5. Vascular Pathology Following the Acute Phase
Heavily impaired blood vessels do not immediately return to normal, even after the acute phase of KD is successfully overcome, and severe acute inflammation is alleviated. The body’s repair mechanisms work slowly to restore original conditions. The time needed for repair is impacted by various conditions, including the extent of damage, the capabilities of the individual's repair mechanism, the presence of repair-inhibiting factors, etc. During this repair period, each patient experiences growth, and some approach adulthood.
At present, there is no consensus concerning the presence or absence of late-stage residual vascular involvement. However, advances in testing methods have revealed evidence of intimal thickening in blood vessels that would have been deemed healthy and normal by conventional testing [16]. In addition, vascular endothelial function testing has recently shown some dysfunction in patients who have a history of KD [17]. There are some reports that the vascular dysfunction may remain to be improved even in chronic phase. Atherosclerotic lesions have also been found in adult KD patients with residual coronary vessel involvement in the chronic phase [18]. Taken together, these reports reflect a growing fear in recent years that KD vasculitis leads to arteriosclerosis.
6. Progression of Post-inflammation Arteriosclerosis or Atherosclerosis and Oxidative Stress
General explanations for the onset and progression of atherosclerosis include Ross’s inflammation response hypothesis [19] and Steinberg’s oxidation hypothesis [20]. Namely, when vascular endothelial cells are damaged, various inflammatory cytokines are released. As a result, activated immunocompetent cells adhere to endothelial cells, then migrate below the endothelium. These activated immunocompetent cells are followed by vascular smooth muscle cells, fibroblasts, and other cells. This combined cellular migration results in further endothelial cell damage. Atherosclerotic changes progress as this vicious circle repeats itself.
Attention has recently been focused on the involvement of oxidative stress caused by ROS accumulation in the progression of these atherosclerotic changes. Oxidative stress triggers inflammation by inducing the expression of adhesion factors and chemokines. This induction occurs via NF-κB activation in vascular endothelial and smooth muscle cells, promoting the formation of atherosclerosis. Superoxides also reduce the expression of vascular endothelial NO synthase (eNOS) and decrease NO production. Decrease NO production triggers contraction of the blood vessel and enhances platelet aggregation, thrombus formation, and intimal proliferation. These factors are believed to exacerbate the progression of vascular endothelial impairment.
7. Oxidative Stress and Impaired Endothelial Function in Chronicphase Kawasaki Disease
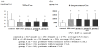
To study the relationship between oxidative stress and sustained vascular endothelial cell damage in chronic-phase KD, we measured urine levels of NOx, which are metabolites of nitric oxide, and urine 8-isoprostane levels, a sensitive marker of oxidative stress (Figure 3). The subjects included 149 patients with a history of KD who had reached puberty and a control group of 367 patients who had no coronary risk factors. The 8-isoprostane levels were notably higher in patients with a history of KD, regardless of whether or not coronary lesions were present. KD patients also showed a significant reduction in NOx, a vasodilation-related factor that originates from vascular endothelial cells. These results suggest the possibility that patients with a history of KD retain some degree of oxidative stress in the chronic phase, and that this oxidative stress is involved in the appearance and progression of vascular endothelial cell impairment.
Thirty-five patients with a history of KD who had reached adulthood were also assessed for endothelial cell dysfunction (Figure 4). The adults with KD showed a clear drop in the endothelium-dependent vasodilation response (%FMD) of forearm arteries, compared with 36patients in a control group. These patients also had a clear simultaneous rise in the level of thrombin-antithrombin complex (TAT), a marker of vascular endothelial cell impairment.
8. Arteroscleroticor Atherosclerotic Changes in Chronic Phase
Chronic-stage vascular endothelial dysfunction and oxidative stress are serious problems for children with a prior history of Kawasaki disease. As these two factors are positively correlated with each other, it is reasonable to hypothesize that they are both involved in the onset and progression of atherosclerosis.
The development of atherosclerosis, also called arteriosclerosis, is a risk factor for coronary events that varies depending on the tissue. Some question the role of atherosclerosis development in KD. The artherosclerotic changes described in actual late-stage patients have often included so-called post-inflammatory arteriosclerosis, which is rich in hyalinized fibrous tissue.
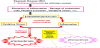
Previously, using an allergic vasculitis model in weaning rabbits exhibiting KD-like vascular lesions, we reported that the induction of vasculitis led to long-term vascular endothelial cell impairment [21]. In this KD-like model, the additional stress of a high-fat diet during the chronic phase, in which vascular inflammation had already improved, caused the atherosclerosis to worsen. This finding suggests that atherosclerosis is readily induced when hyperlipidemia and other risk factors are combined with vasculitis, which is itself a risk factor for atherosclerosis. That is, when other predisposing conditions linked to increased oxidative stress, such as hypertension, diabetes, hyperlipidemia, obesity, smoking, or stress, coexist, atherosclerosis may have an earlier onset in patients with a prior history of KD [22- 26] (Figure 5). Oxidative stress not only seems to trigger post-KD vascular impairment, as is the case for many diseases, but also appears to be a factor in deterioration after onset.9. Antioxidant Therapy and Future Prospects
No clear evidence yet exists that administering antioxidants can mitigate vascular impairment and prevent the onset of artherosclerosis. The incidence of coronary heart disease in France is low despite the high dietary intake of animal fat. This “French paradox” brought attention to the antioxidant action of the polyphenols, which are abundant in red wine [27]. Although subsequent animal research on various antioxidants substantiated their atherosclerosis-inhibiting effect [28], large-scale human clinical trials showed little evidence that they were effective in preventing atherosclerotic disease, and some studies, in fact, showed a tendency toward exacerbation [29,30].
At present, we believe that administering drugs or taking late-stage corrective measures against oxidative stress in children with a prior history of KD may not necessarily immediately improve their vascular prognosis. Despite their cytotoxic effects, ROS play an important role in the biological defense and intracellular signal transduction systems, and their suppression by antioxidant drugs is not necessarily beneficial for the body. Important directions for future research include development of more specifically targeted antioxidant treatment strategies and identification of suitable biomarkers for assessing vascular oxidative stress. Regular examination and early intervention, such as diet and lifestyle guidance, are critical for reducing the risk factors for atherosclerosis in children with a prior history of KD, particularly as they grow older, in order to improve their vascular prognosis. include development of more specifically targeted antioxidant treatment strategies and identification of suitable biomarkers for assessing vascular oxidative stress. Regular examination and early intervention, such as diet and lifestyle guidance, are critical for reducing the risk factors for atherosclerosis in children with a prior history of KD, particularly as they grow older, in order to improve their vascular prognosis.
Competing Interests
The authors declare that they have no competing interests exit.
Author Contributions
All the authors substantially contributed to the study conception and design as well as the acquisition and interpretation of the data and drafting the manuscript.