1. Introduction
Autism spectrum disorders (ASD) are developmental disorders associated with a high individual and social burden, but their aetiology is poorly understood. The prevalence of ASD among 3-17 year-old children has suddenly increased, reaching 2.24% in a 2014 national survey in the United States [1]. The prevalence of ASD is likely to increase in Japan [2].
Vitamin D is a secosteroid associated with peripheral calcium homeostasis and nervous system function [3]. Vitamin D exists in two major forms, vitamin D2 from plants and D3 from animals. Both vitamin D2 and D3 are biologically inert and require activation through two hydroxylation processes involving 25-hydrooxylase (CYP2R1) and 1α-hydroxylase (CYP27B1), located in the liver and kidney, respectively [4]. 1,25-dihydroxyvitamin D (1,25OHD) is a biologically active metabolite produced by two hydroxylation reaction steps in the nervous system [5]. Most activated vitamin D is converted to calcitroic acid [6]. Vitamin D-binding protein transports 85-90% of circulating Vitamin D metabolites in serum [7].
Many cohort studies have shown low neonatal vitamin D to be a possible risk factor for ASD [8,9] . In one case report, Vitamin D supplementation led to significant reduction in core symptoms of ASD [10]. We previously reported that vitamin D supplementation for 9 months might ameliorate typical clinical symptoms in children with autism spectrum disorder [11]. However, to our knowledge there has been no study analyzing vitamin D status after a follow-up period.
The present study was designed to investigate the effects of discontinuation of vitamin D3 supplementation on serum vitamin D status in ASD children.
2. Materials and Methods
2.1 Subjects and setting
Prior to this study, approval was obtained from the ethics committee of Bukkyo University (project registration number in 2018: 7). We enrolled 5 male and 1 female Japanese children with ASD and 5 male typically developing children, all aged 3 years. The researchers were present at the child welfare institution (Mukunokien, Kyoto, Japan) where the study was conducted to assure the proper management of safety and confidentiality in the study. The manager of the institution invited parents to participate in the study, and all the children whose participation was requested from January 2019 to January 2020 were enrolled. All subjects took oral vitamin D supplements (Baby D® 200: 5.0 μg/day of vitamin D3 oil purchased from Morishita Jintan Co., Ltd., Osaka) for 9 months. We recruited typically developing children from among the authors’ acquaintances.
2.2 Serum 25OHD, calcitroic acid and vitamin D binding protein
Blood was collected by venipuncture and serum 25OHD concentrations were measured by Kyoto Microbio Laboratory (Kyoto, Japan). Calcitroic acid and vitamin D binding protein were analyzed using a commercially available chemiluminescent immunoassay kit (Cloud-Clone, Corp., TX, USA ) and ELISA kit (R&D systems Inc., MN, USA), respectively.
2.3 Statistical analysis
The differences between before and after discontinuation and before and after supplementation were evaluated using the Wilcoxon test. Otherwise, we used the t-test. A p-value of <0.05 was considered to be statistically significant. Analyses were carried out using SPSS 21 for Windows (IBM, Japan).
3. Results and Discussion
3.1 Study subjects
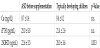
The characteristics of the study subjects are shown in Table 1. Age was 3 years for all the ASD children (males, n=5 females, n=1) and typically developing children (males, n=5). Ca and iPTH were within the normal range, but 25OHD was below the sufficient value (≥30ng/ mL) in ASD children and significantly lower than that in the typical developing children (Table 1).
3.2 Change in serum 25OHD
The 25-hydroxy vitamin D levels were ≥30 ng/mL (sufficient) in 1 child, >20 ng/mL and <30 ng/mL (insufficient) in 4 children and ≤20 ng/mL (deficient) in 1 child after 9 months of vitamin D3 supplementation. No significant difference was seen with levels in the typically developing children (Figure 1a). At 4 months after the end of supplementation, the number of children in the sufficient group decreased from 1 to 0 (1 became insufficient), while the number in the insufficient group decreased from 4 to 2 (2 became deficient). These were the same as the levels prior to vitamin D3 supplementation (Figure 1b).
These results suggest that vitamin D3 supplementation increased serum 25OHD levels, and that they returned to the original levels after the vitamin D3 was discontinued.
Most vitamin D is known to be activated and metabolized to calcitroic acid [6]. In this study, the serum calcitroic acid levels were the same as 25-hydroxy vitamin D during supplementation. At 4 months after the end of supplementation, the serum calcitroic acid level was lower than the level of 25-hydroxy vitamin D. This tendency was similar to the result in typically developing children expect for high25-hydroxy vitamin D levels. These results suggest that supplemented vitamin D3 is quickly converted to calcitroic acid (Figure 2a).
A recent study pointed to the possibility that low vitamin D levels in children with ASD are due to genetic factors associated with vitamin D metabolism [7]. It found the CYP2R1genotype is associated with a higher risk for ASD and with lower circulating form of vitamin D. These results suggest that supplemented vitamin D3 is not effectively transformed to 1,25OHD and metabolized to calcitroic acid. Vitamin D binding protein genetic variability is a significant factor affecting childhood vitamin D status [12]. In this study, no difference was seen in the value of serum vitamin D binding protein levels (Figure 2b).
This is a preliminary study with a very small number subjects. Further study with larger numbers of subjects is warranted, and could reveal optimal 25OHD levels for ameliorating ASD symptoms.
4. Conclusion
These findings indicate that continuous vitamin D3 supplementation is essential for improvement of the low vitamin D status in children with ASD.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Dr. Hasegawa was responsible for the study conception, design,
analysis, interpretation of data, and drafting of the manuscript.
Ms. Mochizuki was responsible for data acquisition and proof
reading of the manuscript, and she participated in the data analysis.
Dr. Yamada was responsible for the data acquisition and proof
reading of the manuscript.
Dr. Morimoto was responsible for the data acquisition and proof
reading of the manuscript.
Ms. Nagaya was responsible for data acquisition and proof reading
of the manuscript.
Acknowledgments
The authors acknowledge Ms. Chizu Shibuya and Ms. Fumie Fukuda for their kind help.