1. Introduction
Breast cancer is the cancer that has the largest effect on procreation in women of reproductive age and its incidence is increasing at an alarming rate. In the US, 1 in every 8 women will develop breast cancer during her life [1]. Chemotherapy plays an important role in breast cancer treatment, and the most recommended chemotherapy treatments for breast cancer in the current guidelines include cyclophosphamide (CPA),Examples in Japan are AC (doxorubicin, CPA), FAC (fluorouracil, doxorubicin, CPA), FEC (fluorouracil, epirubicin, CPA) and EC (epirubicin, CPA). CPA is classified as a group1 agent (carcinogenic to humans) by the International Agency for Research on Cancer and in pregnancy category D by the US Food and Drug Administration Center for Drug Evaluation and Research [2].
The actual exposure of hospital workers to anti-neoplastic drugs in the work environment has been described [3-5]. In 2004, the US National Institute for Occupational Safety and Health (NIOSH) published an alert reviewing the most recent information available and promoting a program of safe handling of these drugs during their use [6]. Although countermeasures for hazardous drugs (HZ) exposure of medical personnel have been developed (JSCN/JSMO/JASPO Joint Guidelines for Safe Handling of Cancer Chemotherapy Drugs, 2015) [7], preventive measures for the families of patients visiting a hospital for treatment or hospitalization are insufficient. Cancer chemotherapy has shifted from inpatient to outpatient settings, and so exposure of medical staff and family members to HZ is a serious concern.
This is a quantitative descriptive study that investigates the kinetics of salivary CPA in order to minimize the risk of CPA exposure after outpatient chemotherapy. Another aim is to document instructions for the prevention of exposure in the homes of the patients receiving outpatient chemotherapy.
2. Materials and Methods
2.1 Subjects and Setting
Prior to the study, approval was obtained from the ethics committee of Ishikawa Nursing University (project registration number 561) and the 2 facilities that carried out the chemotherapy for breast cancer.
We enrolled 31patients (age: 54.7 ± 10.5) who had been diagnosed with breast cancer more than one month previously, had received one cycle of chemotherapy (FEC, EC, CAF, AC treatment protocol including CPA) and had Performance Status (PS)of 0 or 1 (irrespective of age) [8]. The patients were asked to keep saliva for 3 days after outpatient chemotherapy.
Six saliva samples (about 1 mL) were collected at appropriate time intervals (day of chemotherapy: after the chemotherapy and dinner, 2nd and 3rd day: after breakfast and dinner) and stored at -10℃.The frozen samples were mailed to Shionogi Analysis Centre Co., Ltd., Japan and analysed for CPA using LC-MS/MS. The data were fitted to a single-component model, and half-life and elimination rate constant were calculated. Blood data were obtained from medical records.
2.2 Statistical analysis
Results are expressed as the mean ± SD. The differences between patient properties and half-life were evaluated using the Pearson coefficient of correlation. A p-value of < 0.05 was considered to be statistically significant. Analyses were carried out using SPSS 21 for Windows (IBM, Japan).
3. Results and Discussion
3.1 Study subjects
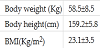
Characteristics of the study subjects are shown in Table 1.
3.2 Change in salivary CPA
The patients received CPA of from 600 to 1200 mg/person (14.5 ± 4.5 mg/Kg). CPA in saliva was 13.6-79.0 (33.1 ± 13.2) mg/mL immediately after the chemotherapy, and then exponentially decreased with the passage of time (half-life of 6.62 ± 1.24 h, elimination rate constant of 0.171) (Figure 1). These values were slightly lower than that determined from saliva in male patients undergoing chemotherapy (half‐life of 8.38 ± 2.25 h, elimination rate constant of 0.275) [8]. These results suggest that there are no sex differences in the elimination of CPA. Salivary CPA kinetics (half-life) was found to be slightly lower than plasma CPA kinetics (half‐life of 7.61 ± 4.1 h) in breast cancer patient [9]. These results indicate that the noninvasive method of saliva sampling may be a useful approach in monitoring CPA concentration in patients.
The inset illustrates salivary CPA kinetics. The values are expressed as % of CPA immediately after the chemotherapy.
We estimated CPA to be about 15-87% after dinner on the day of chemotherapy, 2.0-18.9% after breakfast and 0.08-5.09% after dinner on the 2nd day, and 0.02-0.6% after breakfast and 0.002-0.1% after dinner on the 3rd day. It took one and a half days (36 h) to minimize the exposure risk to family members after the patient had received outpatient chemotherapy.
Breast cancer is the most commonly diagnosed cancer in women worldwide and its accounts for 29% of all new cancers in women. Its onset age is also younger than that of other cancers. It is important for patients and their family members to protect themselves from salivary unaltered CPA. During occupational exposure, the contamination can take place via respiratory and/or cutaneous and/or oral routes [2]. A better understanding of how to use chopsticks in cooking, eating and feeding children when the chopsticks have touched the patient’s mouth, and how to wash toothbrushes or clothes, is urgently needed to protect others from salivary CPA during the one and a half days after outpatient chemotherapy. A previous study found that the total amount of CPA excreted in the urine during the 48 h postadministration period was 24.3% of the total administered dose in breast cancer patients [10]. The patient and family members also need to be careful of contamination of the toilet environment.
3.3 Liver function
CPA exhibits a number of toxic effects, including hepatotoxicity, and nephrotoxicity [11]. A significant positive correlation was observed between AST and T1/2 (γ = 0.393, P < 0.05). It is necessary to minimize mental and physical stress to protect liver function.
4. Conclusion
These findings show that it takes one and a half days after outpatient chemotherapy to minimize the exposure risk to patients and family members.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Dr. Makino was responsible for the study conception, design, data
acquisitionand proof-reading of the manuscript.
Dr. Hasegawa was responsible for the study conception, design, and
analysis, interpretation of data, and drafting of the manuscript.
Ms. Takizawa was responsible for data acquisition and proof-reading
of the manuscript, and she participated in the data analysis.
Ms. Matsumoto was responsible for proof-reading of the manuscript,
and she participated in the data analysis.
Dr. Wagatsuma and Ms. Yabushita were responsible for data
acquisition and proof-reading of the manuscript.
Dr. Kubo and Dr. Aogi were responsible for the study design and
proof-reading of the manuscript.