1. Introduction
The incidence of tick borne diseases is on the rise. These trends result in larger numbers of persons requiring treatment, placing a greater financial impact on the healthcare system and individual patients thus creating a greater burden on society. Prevention measures, pharmacological treatment, possible vaccines, and nonpharmacological interventions such as behavior changes or ticktargeted strategies need to be evaluated and improved. Interventions need to include emphasis on public health education [1].
Lyme disease is the most common vector borne disease in North America and Europe with approximately 300,000 cases per year in the U.S.[2] The spirochete infection is caused by the Borrelia species (B. bourdorferi, a spirochete related to the syphilis pathogen) and is transmitted by the bite of infected Ixodes ticks (Ixodes scapularis also known as deer ticks or black legged ticks and Ixodes pacificusalso known as western black legged ticks)[2]. Lyme disease can affect the skin, joints, nervous system, and heart. In the U.S., the Ixodes ticks can also transmit human granulocytic anaplasmosis, babesiosis, and Powassan virus [2,3]. Infected Amblyomma americanum (Lone star ticks) can transmit human monocytic ehrlichiosis and STARI (Southern Tick Associated Rash Illness). Also, lone star larval tick bites can cause an allergy to meat due an IgE immune response to galactose-alpha-1,3-galactose also known as alpha gal [4]. The Dermacentor variabilis tick can transmit Rocky Mounted Spotted Fever (RMSF), tularemia and tick paralysis. Pathogenic organisms normally cycle among small mammals and birds. The white-footed deer mouse (Peromyscus leucopus) is the dominant mammalian reservoir host for tick borne diseases, especially Borrelia burgdorferi. It is estimated in some geographic regions (North Eastern U.S.) over ninety percent of the white footed deer mouse are infected [5].
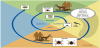
Ticks have three stages in their two-year life cycle: larva, nymph, and adult. Lyme disease is most commonly transmitted by nymphal ticks, which are most active during the late spring and early summer in temperate regions. Nymphal ticks are smaller in size and not as easily detected on skin. Larger adult ticks are most active in the fall. (Figure 1) [6,7].
Individuals at highest risk for tick borne diseases including Lyme disease are those who live in endemic regions. Individuals in endemic areas who work outside and/or participate in recreational activities are at even higher risk for tick borne diseases. Confirmation of tick borne diseases may be based on laboratory testing; however, antibiotic therapy should not be delayed in a patient with suggestive clinical presentation [2].
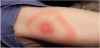
Early cutaneous infection with B. burgdorferi can manifest with a bull’s eye rash also known as erythema migrans (EM). Appearance of rash may occur less than fifty percent in Lyme disease infections. Ticks can transmit more than one disease to humans resulting in coinfections such as Lyme disease and babesiosis or Lyme disease and ehrlichiosis [2,3].
2. Life Cycle of Hard Ticks
After hatching from eggs, ticks go through three stages which include larval, nymphal, and adult stages. Ticks must have a blood meal at each of these three stages to survive and will die if they do not find a host for their next feeding. (Figure 2)[7,8].
Ticks find their animal hosts by sensing their body heat, vibration and/or carbon dioxide. Hosts consist of mammals such as deer, horses or humans. Preferred hosts for larval ticks consist of rodents such as mice, squirrels and chipmunks. Larval ticks also feed on birds. If the rodents or birds are infected with a tick-borne disease such as Borrelia burgdorferi, the tick then becomes infected during feeding. Nymphal and adult ticks prefer larger hosts such as deer, horses, dogs or humans. Larger mammals including deer and humans are considered dead end hosts (infection cannot be transmitted to feeding ticks) [7,8].
Ticks that need a blood meal wait for their host by resting on tips of grasses or shrubs. They can also wait in leaf litter. Ticks do not jump or fly, but wait with hind legs attached to vegetation known as “questing”.
When a host brushes by the positioned tick it quickly attaches to the skin of the host. The tick attaches into skin with their barbs that anchor into the skin. Ticks also secrete cement like substance to further secure their anchor along with an anesthetic chemical in their saliva so their presence can go unnoticed by the host [6-8] .
The longer an infected tick is attached and feeds the more likely the transmission of disease, especially if the tick becomes engorged. Ticks that feed on humans, especially nymphal ticks can become attached, feed and then fall off without any awareness of the individual, especially if ticks are attached in areas such as the groin, axillae, back or behind the knees [7,8]. Ticks feed by sucking the host's blood for several days. If the host reservoir such as a rodent or bird is infected, the tick then becomes infected. The next time the tick feeds in its life cycle, the tick then may infect an animal or human. It is estimated that twenty percent of nymphal Ixodes scapularis and fifty percent of adult Ixodes scalpularis are infected with Borrelia burgdorferi [7,8].
Tick eggs are not initially infected; however, when eggs molt into larva and they feed on an infected rodent such as a mouse, then the tick become infected. The larval tick then molts into a nymph; the infected nymphs seek a blood meal which can be a deer or human and then transmits the infection during the feeding process. The adult ticks prefer to feed and mate on white tailed deer (Odocoileus virginianus), with females then falling off to lay their eggs before they die. A single female tick typically lays one to three thousand eggs. White-tailed deer are the principal host for the adult ticks; an important means of transport and tick abundance is closely linked to the abundance of these animals. A frost does not kill ticks and adults may become active as soon as it is above freezing [9].
One key success of I. scalpularis as a Borrelia vector relies on its ability to limit proliferation of spirochetes. Certain gene expression contributes to the innate ability of I. scapularis to control B. burgdorferi levels after its acquisition. This has potential ramifications for Lyme disease transmission, as spirochete load in the tick can influence transmission efficiency [10].
3. Environmental Factors
Factors that foster disease prevalence in ticks and risk of disease for humans include environmental influences which include precipitation, abundance of acorn production in areas in the northeastern US. The abundance of acorns influences white-footed deer mouse and deer populations [5].
Reshaping of landscapes caused by humans has set the change in the ecology of Lyme disease and other tick borne diseases. By the middle of the 20th century farming became predominant in the Mid- Western United States which forced the farms to close in the northeast allowing fields to become mixed hard wood forests. Encroachment of suburban environments facilitated increase in white-tailed deer as they were no longer sought after for food [5,6].
These peri-domestic changes have drastically reduced medium sized predators that rely on rodents in the food chain, resulting in increased numbers of the white-footed mouse population who are competent reservoirs for tick borne diseases. [5]This paradigm can be applied to other areas of North America and Europe.
White tailed deer are heavily covered with ticks in the late fall and early spring. The use of roller applicators such as the “four posters” which apply insecticides to the deer while they are eating corn placed into bins. The pesticides then kill the adult ticks which then halt the ticks from laying of eggs, therefore reducing tick populations [1].
4. Lyme Disease
Lyme disease early signs and symptoms can occur three to thirty days after a tick bite. Signs and symptoms may elusive. Predominant in spring through summer, symptoms can include flu like symptoms such fever, chills, headache, myalgias, joint pain and swollen lymph nodes. An erythema migrans (EM) rash (Figure 3) may appear at the site of the tick bite three to thirty days (average of 7 days) this rash may then expands over a period of days and can reach up to 30 cm in size which may clear as it enlarges to appear as a target or a “bull’s eye configuration.” If a rash does appear it may not always have a classic EM appearance. A rash may not always be apparent in darker skinned individuals, a rash may appear as a bruise-like. Although the CDC in 2015 reported a rash in 70% of Lyme cases, this is often not the case. Fever and generalized signs and symptoms may occur without a rash. Presence of a rash makes the diagnosis of Lyme easier, but disease cannot be ruled out if a rash is not present [2,3].
Later signs and symptoms, especially if Lyme disease is initially missed, can occur days to months after a tick bite. These symptoms can have overlap in early and late Lyme disease. Symptoms include severe headaches, neck stiffness, visual changes, mental cloudiness, fatigue, arthritis with severe pain and swelling commonly of knees and other large joints (Lyme arthritis), myalgia, bone and or tendon pain, heart palpitations (Lyme carditis), facial palsy, nerve pain, neuropathy, shortness of breath, malaise. Additional EM rashes may appear on other parts of the body. Also, in addition to headache, neck pain and fever, meningeal signs or meningitis (inflammation of meninges of the brain and spinal cord) symptoms can include changes in mental status, dizziness and short term memory impairment [2,3].
In children, a sudden onset of symptoms may occur such as severe fatigue unrelieved by rest, insomnia, joint pain, headaches, nausea, abdominal pain, difficulty with concentration and learning (often mis-diagnosed with attention deficit disorder or other behavioral problems) or even food aversions. Early symptoms may be missed or even dismissed in children especially if a rash is not noticed or present. Children are especially vulnerable to tick-borne diseases because they are physically close to the ground during activities and play [7].
A small bump or redness at the site of a tick bite that occurs immediately and resembles a mosquito bite is common. This irritation generally goes away in 1-2 days and is not a sign of Lyme disease. A rash with a very similar appearance to EM occurs with Southern Tick-associated Rash Illness (STARI), but is not Lyme disease. Ticks can spread other organisms that may cause a different type of rash [2].
4.1 Treatment
Treatment includes oral antibiotic of doxycycline course (100mg twice daily) for 14-21-dayduration. Dosing can be extended for 28 days based on symptoms. Doxycycline should be taken with food (nondairy) to prevent GI upset. Sun safety is important to prevent photosensitivity. Adults who may be allergic to doxycycline or work outside should be given amoxicillin. Long courses of doxycycline are not indicated for children less eight years of age due to the potential of teeth staining. Amoxicillin dose is weight based for children and the usual duration of treatment in 21 days. It is important for completion of antibiotic course to effectively eliminate the infection. Neurological Lyme disease is diagnosed by spinal fluid analysis and requires an extended course of intravenous ceftriaxone [2,3].
It is not uncommon for patients treated for Lyme disease with a recommended 2 to 4-week course of antibiotics to have lingering symptoms of fatigue, pain, or joint and myalgias at the time they finish treatment. In a small percentage of cases, these symptoms can last for more than 6 months. Although sometimes called “chronic Lyme disease,” this condition is properly known as “Post-treatment Lyme Disease Syndrome” (PTLDS). The exact cause of PTLDS is not yet known. Most experts believe that the lingering symptoms are the result of residual damage to tissues and the immune system that occurred during the infection. Similar complications with auto–immune like responses are known to occur following other infections. B. burgdorferi does not remain in the blood so culturing is problematic. More research needs to be done to determine if the spirochetes are still present as the organism can wall off into cyst like forms in collagen rich tissue [3].
For prevention after a recognized I. scapularis tick bite a single prophylactic dose of 200mg of doxycycline may be given to adults and children greater than eight years or older based on the following criteria: the nymphal or adult tick has been attached for greater or equal to 36 hours and the tick is engorged [3].
4.2 Diagnostic Testing
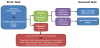
Laboratory diagnosis includes IgM or IgG antibodies in serum. A two-tier testing protocol EIA or IFA is performed first. If positive or equivocal it is followed by a Western blot. Polymerase chain reaction (PCR) may also be done. The two-tier test for antibodies is usually negative in early Lyme disease. (Figure 4) It may take up to one month for seroconversion to occur. If a patient is treated early with appropriate antibiotics, antibodies may not be present. PCR may be negative as the spirochete prefers to migrate into tissues or joints. In suspected Lyme meningitis testing for intrathecal (requiring a spinal tap) IgM or IgG is recommended [2,3].
4.3 Lyme Vaccine
Currently there are no tick-borne disease vaccines for humans in the United States. In 1998, the U.S. licensed a vaccine for Lyme disease; however this vaccine was withdrawn from the market in 2002. This vaccine was based on an outer surfacecell protein A (Osp A) found on the pathogen which prevented transmission of Borrelia burgdorferi from ticks to humans by killing spirochetes in ticks. Three doses were needed to provide protection from infection (only 80 percent protection). Unfortunately, the problem was that antibody titers did not persist for long periods of time requiring frequent boosters. Additionally, there were reported autoimmune related side effects, including arthritis and neuropathology causing its elimination. Research goals are to design an anti-tick saliva vaccine aimed to reduce prolonged tick attachment [1].
4.4 Babesiosis
Babesiosis is most frequently reported from endemic areas of the North Eastern and upper Mid-Western U.S. Like malaria, the organism is a parasite: Babesia microti which infects red blood cells. The incidence of babesiosis is approximately 1,700 cases reported to the CDC from 2001-2014 and is on the rise. This organism can also be transmitted from infected blood donors. Babesia infection can range from anasymptomatic to a life threatening clinical course. Increased risk factors for severe babesiosis include asplenia, impaired immunity and advanced age. Severe cases can be associated with marked thrombocytopenia, disseminated intravascular coagulation, end organ failure and death [2,3].
The incubation period for babesiosis is one to nine weeks or longer. Signs and symptoms can be like Lyme disease to include constitutional symptoms such as fever, chills, diaphoresis, fatigue, malaise, joint pain and myalgias. Gastrointestinal symptoms can include nausea and anorexia. Splenomegaly and hepatomegaly can also occur [2,3].
4.5 Diagnostic testing
Identification of intraerythrocytic babesia parasites by lightmicroscopy of a peripheral blood smear typically has a “Maltese cross” appearance seen on red blood cells. Other diagnostic tests such as polymerase chain reaction (PCR) analysis for Babesia microti and indirect fluorescent antibody (IFA) for Babesia-specific antibody titer may be routinely done. General laboratory findings may show decreased hematocrit, thrombocytopenia and elevated liver transaminase values. [2,3] It is important to note that in endemic areas babesia co-infection may occur with Lyme disease. It is important to consider testing to rule out possible babesiosis as drug treatment is different than in Lyme disease [3].
4.6 Treatment
Treatment of babesiosis requires a combination of oral atovaquone (750 mg every 12 hours) and azithromycin (500-1000mg daily) for seven to ten days. Also, a combination of intravenous clindamycin and oral quinine can be given for seven to ten days in patients with more severe symptoms. Occasionally, exchange transfusions may be required for critically ill patients. [2,3].
4.7 Anaplasmosis
Anaplasmosis (Anaplasma phagocytophilum) which is transmitted by the Ixodes Scapularis tick is most frequently reported in the northeastern and upper Midwest areas of the U.S. that correspond to the geographic areas endemic for Lyme disease. The incidence of anaplasmosis reported by the CDC was approximately 1,700 in 2010. [2,3].
The incubation period for anaplasmosis is one to two weeks. Clinical signs and symptoms are like other tick borne diseases such as ehrlichiosis such as fever, chills, malaise, myalgia, GI symptoms, cough and rarely a rash [2,3].
4.8 Diagnostic testing
Antibodies to A. phagocytophilum are usually detected seven to ten days after illness onset. Positive laboratory results show a four-fold change in IgG specific antibody titer by immunofluorescence assay (IFA) in paired serum samples (second sample taken two to four weeks later). Also, positive detection of DNA polymerase chain reaction (PCR) is most sensitive within the first week of illness. Other lab findings associated with anaplasmosis are leukopenia, elevated hepatic transaminases and anemia. Visualization of morulae in the cytoplasm of granulocytes may be seen; however, blood smear examination is insensitive and should not be relied on solely to rule out anaplasmosis [2,3].
4.9 Treatment
Recommended treatment of anaplasmosis is Doxycycline 100mg mg twice per day, orally or intravenously for a minimum of 5-7 days. Doxycycline is also recommended for children (weight based dosing of 2.2mg/kg twice per day). (CDC, 2015) Use of antibiotics other than doxycycline increases the risk of death. There has been no evidence shown causing tooth staining at the recommended dosing and duration in children less than eight years of age [11].
4.10 Ehrlichiosis
The areas from which cases are reported correspond to the known geographic distribution of the lone star tick (Amblyomma americanum) which transmits both E. chaffeensis and E. ewingii. The incubation period is one to two weeks. Ehrlichiosis and anaplasmosis have a similar clinical presentation such as fever, headache, malaise, myalgia and GI upset. In children, a rash may occur [2,3].
4.11 Diagnostic testing
Antibodies to Ehrlichia are usually detected seven to ten days after illness onset. Positive laboratory results show a four-fold change in IgG specific antibody titer by immunofluorescence assay (IFA) in paired serum samples (second sample taken two to four weeks later). Also, positive detection of DNA polymerase chain reaction (PCR) is most sensitive within the first week of illness. Other lab finding associated with ehrlichiosis are leukopenia, elevated hepatic transaminases and anemia. During the acute stage of illness, morulae (clusters of Ehrlichia multiple in monocyte vacuoles to form large mulberry shaped aggregates) may be detected in in twenty percent of E.chaffeenis cases [2].
4.12 Treatment
Doxycycline 100mg mg twice per day, orally or intravenously for a minimum of 5-7 days (usual oral dosing7-14 days) is the recommended first line of treatment for ehrlichosis. Doxycycline is also recommended for children (weight based dosing of 2.2mg/kg twice per day) [2]. Use of antibiotics other than doxycycline increases the risk of death. There has been no evidence shown causing tooth staining at the recommended dosing and duration in children less than eight years of age [11].
Lone star tick larvae are very active during the late summer and early fall. They are voracious feeders which feed multiple times. Exposure to larval tick bites may cause a delayed allergic reaction to meat due to antibodies to Galactose-alpha-1,3-galactoseor commonly known as alpha gal [4].
Alpha-gal is not naturally present in humans (or apes), but is present in all other mammals. Lone star eggs contain alpha-gal carbohydrate. Lone star larval ticks can then inject the alpha-gal into a person's skin, which in turn will cause the immune system to release a flood of IgE antibodies to fight off the foreign carbohydrate [4].
4.13 Treatment
An affected individual with alpha gal allergy typically experiences an allergic reaction three to six hours after ingesting mammalian meat such as beef, pork or lamb. Allergic reaction symptoms may be worse with consumption of fattier meats. Symptoms range from pruritus, GI upset to angioedema [4].
There is a diagnostic test for antibodies to alpha gal. Patients with this allergy need to be under the care of an allergist, avoid meat, meat products and gelatin. An epinephrine auto injector is also needed for possible anaphylactic reactions. This allergy can wane over time if there is no re-exposure to Lone star larval tick bites [4].
5. Rocky Mounted Spotted Fever
Rocky Mounted spotted fever (RMSF) has been reported in all continuous U.S. states. However, sixty percent of cases have been reported from North Carolina, Arkansas, Tennessee, Oklahoma and Missouri and more recently in Arizona. The incidence of RMSF has increased to 8.4 cases per million persons from 1.9 cases per million in 2000. RMSF can be rapidly fatal if not treated within the first five days of symptoms. RMSF is caused by Rickettsia rickettsii transmitted by an infected Dog Tick (Dermacentor variabilis)[2].
The incubation period for RMSF is one to two weeks. Clinical symptoms consist of fever, chills, severe headache, malaise, myalgias, GI upset, cough, photophobia, focal motor nerve paralysis or sudden transient deafness. A maculopapular rash typically appears two to five days after the onset of fever. Rash typically is non-pruritic, appears initially on the wrists, forearms, and ankles which then spreads to the trunk and occasionally to the palms and soles. Approximately ten percent of all RMSF never develop a rash or a rash may develop later in disease [2].
5.1 Diagnostic testing
Antibodies to R. rickettsii are usually detected seven to ten days after illness onset. Positive laboratory results show a four-fold change in IgG specific antibody titer by immunofluorescence assay (IFA) in paired serum samples (second sample taken two to four weeks later). Detection of Rickettsia rickettsii DNA in a skin biopsy of the rash by Polymerase Chain Reaction assay (PCR) confirms the diagnosis. PCR of blood is generally unreliable. Other lab findings associated with RMSF are thrombocytopenia, elevated hepatic transaminases and hyponatremia [2].
5.2 Treatment
Doxycycline 100mg mg twice per day, orally or intravenously for a minimum of 5-7 days is the recommended first line of treatment. Doxycycline is also recommended for children (weight based dosing of 2.2 mg/kg twice per day)[2]. Use of antibiotics other than doxycycline increases the risk of death. There have been no evidence shown causing teeth staining at the recommended dosing and duration in children less than eight years of age [11].
5.3 Tularemia
Tularemia (Francisella tularensis) can be transmitted by the dog tick (Dermacentor variabilis), wood tick (Dermacentor andersoni) and the lone star tick (Amblyomma americanum). Tularemia can also be transmitted from contact or in the handling of infected animals such as rabbits [2].
Incubation period for Tularemia is usually three to five days. Clinical symptoms may include fever, chills, headache, malaise, anorexia, vomiting, diarrhea, abdominal pain, myalgia, chest discomfort, cough, sore throat, photophobia, excessive lacrimation, conjunctivitis, lymphadenopathy. There may a cutaneous ulcer at infection site (not always present) with localized lymphadenopathy [2].
6. Diagnostic Testing
Positive laboratory results show a four-fold change in IgG specific antibody titer by immunofluorescence assay (IFA) in paired serum samples or a single antibody titer. General laboratory findings include elevated sedimentation rate and leukocyte count, thrombocytopenia, hyponatremia, elevated hepatic transaminases, elevated creatine phosphokinase, myoglobinuria or sterile pyuria [2].
6.2 Treatment
Treatment for tularemia includes regimen of streptomycin (intramuscular), gentamycin (intramuscular or intravenously), oral ciprofloxacin or doxycycline. Regimens range from 10-21 days based on severity of disease. Children are treated by weight based doses of streptomycin, gentamycin or ciprofloxacin. (Gentamycin or streptomycin is preferred for treatment of severe tularemia which needs to be adjusted for renal insufficiency). Chloramphenicol may be added to streptomycin to treat meningitis [2].
6.3 Powassan Virus Disease
Powassan virus (POW virus) infections have been recognized in the U.S., Canada and Russia. In the U.S. cases are primarily from the northeastern and great lake regions. Powassan virus which is a flavivirus, can be transmitted by the deer tick (Ixodes scapularis). Approximately 75 cases of POW virus cases reported in the U.S. over the past ten years [2,12].
The incubation period is one to four weeks. Clinical symptoms include fever, headache, vomiting and generalized weakness. Symptoms usually progress to encephalitis and/or meningitis with altered mental status, seizures, aphasia, paresis, movement disorders or cranial nerve palsies. Approximately have of survivors have permanent neurologic sequelae with ten percent of POW virus encephalitis cases are fatal. Individuals with POW are often in the Intensive Care Unit requiring ventilator and hemodynamic support [2,12].
6.4 Diagnostic testing
There are no commercially available tests. Testing is available at the CDC and in selected state health departments. IgM antibodies can be detected in serum or cerebrospinal fluid (CSF). However, cross reactivity can occur with other flavivirses such as West Nile, or dengue fever [2].
6.5 Treatment
There is no specific antiviral treatment. Patients often require supportive care in the Intensive Care Unit especially if ill with complications of encephalitis or meningitis [2].
7. Southern Tick Associated Rash Illness (STARI)
Since the 1980s, in the south central and southeastern US, patients have been observed to have Lyme disease-like rashes on patients after exposure to tick bites. However, the tick vector associated with these lesions is the Lone Star tick (Amblyomma americanum), rather than either of Ixodes tick species – I. scapularis or I. pacificus which are known to transmit Lyme disease in the United States. It is unclear if Borrelia burgdorferi, is associated with these rashes. This clinical entity has been differentiated from Lyme disease and is called Southern Tick-Associated Rash Illness, or STARI [13].
The hallmark of Southern Tick-Associated Rash Illness is the Lyme-like lesion. The rash usually appears within seven days of a Lone Star tick bite, and similar to the Lyme lesion, expands in a circular or elliptical fashion. Patients with STARI can also have constitutional symptoms, such as fever, headache, stiff neck, myalgia’s and joint pain, but these are less frequent and generally less severe in STARI patients than in patients with Lyme disease [13].
7.1 Diagnostic testing
The causative agent of STARI has never been cultured and is not currently known. However, some evidence exists that a recently discovered spirochete, Borrelia lonestari, may be responsible: B. lonestari has been detected by polymerase chain reaction (PCR) in Lone Star ticks removed from humans, as well as in Lone Star ticks collected during general epidemiological studies [13].
7.2 Treatment
Tick Removal needs to be done as soon as a tick is noticed. Tick removal techniques from adults and children would be the same for domestic animals such as cat, dogs and horses (figure 5) [2].
Education for proper tick removal should include:
- Use of fine-tipped tweezers to grasp the tick as close to the skin's surface as soon as possible.
- Pull tick upward with steady, even pressure.
- Don't twist or jerk the tick; this can cause the mouth-parts to break off and remain in the skin. If this happens, remove the mouth-parts with tweezers.
- If unable to remove the head easily with clean tweezers, area can be left alone to let/or the skin heal. Transmissions of pathogens do not occur if head of tick remains in skin, however this can result in skin irritation and inflammation.
- After removing the tick, thoroughly clean the bite area and hands with rubbing alcohol, an iodine scrub, or soap and water.
- Avoid following folklore remedies such as the use of hot matches; petroleum jelly or nail polish remover. These interventions are not effective can cause injury or infection.
- Dispose of a live tick by submersing it in alcohol, placing it in a sealed bag/container, wrapping it tightly in tape, or flushing it down the toilet [2].
7.3 Prevention of tick exposure
It is important for the public to be educated in taking preventive measures against ticks year-round and to be especially vigilant in warmer months (April-September) when ticks are most active. Avoidance of wooded and brushy areas with high grass and leaf litter will reduce exposure risk [2].
Education should include
- Walking in the center of trails, avoiding brushing against tall grass and shrubs.
- Use of repellents that contain 20 to 30% DEET (N, N-diethyl-mtoluamide) on exposed skin for protection that lasts up to several hours. Always follow product instructions. Parents should apply this product to their children, avoiding hands, eyes, and mouth.
- Use products that contain permethrin on clothing. Treat clothing and gear, such as boots, sneakers, pants, socks and tents with products containing 0.5% permethrin. It remains protective through several washings. Pre-treated clothing is available and may provide longer-lasting protection.
- Bathing or showering soon after returning indoors (preferably within 2 hours) to wash off and more easily find ticks.
- Conduct a full-body tick check using a hand-held or full-length mirror to view all parts of body upon returning from tickinfested areas.
- Parents should check their children for ticks under the arms, in and around the ears, inside the umbilicus, behind the knees, between the legs, around the waist, and especially in their hair.
- Examine gear and pets. Ticks can ride into the home on clothing and pets, then attach to a person later, so carefully examine pets, coats, and day packs.
- Tumble dry clothes in a dryer on high heat for 10-20 minutes to kill ticks on dry clothing after returning indoors. If the clothes are damp, additional time may be needed. If the clothes require washing first, hot water is recommended. Cold and medium temperature water will not kill ticks effectively. If the clothes cannot be washed in hot water, tumble dry on low heat for 90 minutes or high heat for 60 minutes [2].
To reduce tick exposure near home environments, it is helpful to:
- Clear tall grasses and brush around home and at the edge of lawns.
- Maintain a three-foot barrier of wood chips or gravel between lawns and wooded areas and around patios and play equipment. This will restrict tick migration into recreational areas.
- Frequent moving of lawns: grass should be kept at three inches or less in height.
- Remove leaf litter (ticks do not survive in dry, hot, low humid conditions; they prefer to stay in shaded, damp areas such as in leaf litter).
- Keep wood neatly stacked in a dry area as this discourages rodents that ticks feed on.
- Keep playground equipment, decks, and patios away from yard edges and trees and place them in a sunny location, if possible. Remove trash from the yard that may give ticks a place to hide.
- The use of acaricides (tick pesticides) can reduce the number of ticks in treated areas of outdoor living spaces and yards.
- The Environmental Protection Agency (EPA) and state (US) determine the availability of pesticides. There are professional pesticide companies that can apply appropriate pesticides [2].
8. Conclusion
Tick-borne diseases should be focused on preventative measures similar to other infections of public health significance. Environmental interventions to reduce mouse and other animal reservoir populations, reduction in deer and tick populations need to be implemented and expanded. Research with increased funding is essential for improved and timely diagnostic tests. Research is also needed for a safe vaccine, especially for Lyme disease. Continued evidenced based practices need to be on the forefront. Tick analysis for prevalence of infection in endemic areas needs to be expanded, to include identification of new emerging pathogens. Prevention measures to limit exposure offers the best strategy over the long term to reduce the burden and impact of tick-borne infections [1].
Competing Interests
The author declare that there is no competing interests regarding the publication of this article.