1. Introduction
In the twenty-first century, electronic and information engineering has been highly developing, and portable electronic devices has been indispensable to modern life. With enhanced energy and power densities, lithium ion battery (LIB) has replaced the conventional batteries in the market for portable electronic devices. Nowadays LIB has been widely applied in electric vehicles (EVs) and hybrid electric vehicles (HEVs), especially in energy storage devices [1-4]. In order to meet the demand of human beings for high capacity and long service life, intensive efforts are still underway to further improve this battery.
As an important component of the battery, the electrolyte is one of the key factors that affect the performance and the cost of LIB. Ethylene carbonate (EC) becomes an essential electrolyte solvent since it can form an effective solid electrolyte interphase (SEI) initiated by the ring opening. And it is easy to be influenced by the surface properties of graphite [5]. Generally, carbonate-based electrolyte significantly decomposes at high voltage (>4.5 V vs. Li/L+i), resulting in relatively poor cycling performance for high-voltage LIB [6]. As electrolyte salt, LiPF6 is widely used in commercial LIB. And it has been reported to react with electrolyte solvent. Solvent can induce and accelerate the decomposition of LiPF6: LiPF6→LiF+PF5, the produced PF5 not only reacts with the solvent, but also catalyzes solvent polymerization. But some compounds can decrease the reaction activity of PF5 by -C=O or -P=O weak binding effect [5,7-9].
One of the most economic and effective methods to overcome the above problem and to improve LIB performance is addition of electrolyte additives. The amount of an additive in the electrolyte is less than 5% either by weight or by volume; its effect on the cycleability and cycle life of LIB is significant. The electrolyte additives were discussed and summarized by researchers such as Zhang S.S. [7]. More specifically, diverse additives such as Li2CO3, vinylene carbonate (VC) have been reported as effective additives to improve the cycle life of LIB [10,11].
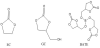
Glycerol carbonate (GC) molecule has the same ring structure as EC shown in Scheme 1. And hydroxyl group provides a facile way to introduce various functional groups. Boron-containing compounds have been found to be effective electrolyte additives due to the lack of electrons in boron atom, and the film formed by boron-containing compounds can stabilize anion PF6- to prevent the formation of the detrimental anion F- and to suppress the electrolyte further decomposition [2,12]. To combine EC structure with boron element, boric acid tris-(2-oxo-[1,3]dioxolan-4-ylmethyl) ester (BATE) was designed for possession of their advantages (BATE chemical structure was shown in Scheme 1). And this new boron-containing compound was carefully studied as electrolyte additive in this work.
2. Experimental
2.1 Additive preparation
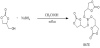
Glycerol Carbonate (GC), Sodium Borohydride (NaBH4), Acetic acid (CH3COOH) purchased from Jiangtian Chemical Technology Co., Ltd., Tianjin, China were analytically pure. To synthesize BATE, in a carefully dried 100 mL 3-neck round-bottom glass flask equipped with a magnetic stirrer, a reflux condenser and a pressure equalized dropping funnel, GC (17.71 g, 0.15 mol) and NaBH4 (1.90 g, 0.05 mol) were placed. CH3COOH (2.86 mL, 0.05 mol) was added dropwise to the slurry over a period of 15 minutes. The reaction mixture was then heated to reflux temperature for an additional 4 h until no more hydrogen was generated. After reaction, a certain amount of anhydrous dichloromethane and ethanol were added to the mixture and the precipitation was filtrated. The filtrate was evaporated under reduced pressure to give the product without further purification. BATE used in our work was synthesized by the parent compound GC in the reaction presented in Scheme 2.
2.2 Electrolyte preparation
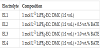
1mol L-1 LiPF6-EC: DMC (1:1 vol., Lithium Battery-grade) used in this work was received from Guangzhou Tinci Materials Technology Co., Ltd.. Considering the solubility and the effects of the compound on the cell electrochemical performances, electrolytes with the composition shown in Table 1 were prepared in an Ar-filled glove box (Super 1220/750/900, Mikrouna Mech. Tech. Co., Ltd., Water content: <1ppm, oxygen content: <1ppm).
2.3 Electrode preparation
Materials were obtained from Huizhou Hyperpower Batteries Inc.. Prior to use, both the Mesocarbon microbeads (MCMB) and Super-P carbon (SP) were dried at 100°C under vacuum condition for about 10 h. To prepare the electrode, a slurry consisting of uniformly mixed 90 wt.% active material (MCMB), 5 wt.% conductive additive (SP), and 5 wt.% polyvinylidene fluoride (PVDF) as a binder dispersed in 1-methyl-2-pyrrolidinone (NMP) was spread onto a 10μm thick copper foil current collector and dried overnight under a reduced pressure at 120°C. The obtained electrode sheets were compressionmolded using a rolling press machine to form the active layer and punched to 13 mm in diameter. The active layer thickness was about 50 μm.
2.4 Electrochemical measurements
To evaluate the electrochemical properties of the electrolytes, Li/ graphite half-cells (CR2025, 20 mm in diameter and 2.5 mm in thickness) were assembled in the argon-filled glove box. Each cell consisted of a MCMB working electrode, a micro porous polyolefin separator, and a lithium metal foil (0.4 mm in thickness, 15.8 mm in diameter). Charge-discharge was carried out under a constant current rate of 0.1C using a battery testing device (Neware Electronic Co. Ltd.) interfaced to a computer with software in the potential range between 0.005 V and 2.000 V (vs. Li/Li+) at 25°C. Electrochemical impedance spectroscopy (EIS) of the cells after 2 cycles and 20 cycles were measured with an electrochemical workstation (IM6e, Zahner), data were collected in the potentiostatic mode in the 100 kHz-10 mHz frequency range at an amplitude of 5 mV. The cells were taken apart after charge-discharge test, and the MCMB electrodes were soaked in and rinsed with acetone to remove the remainder electrolyte. The morphologies of the electrodes after dried in a vacuum drier were observed by scanning electron microscope (KYKY-EM3200A, KYKY Technology Co. Ltd.). The cyclic voltammetry (CV) measurements of coin cells were conducted using a Princeton Applied Research Potentiostat/Galvanostat Model 263A electrochemical analyzer. CVs were measured within the voltage range from 0.005 V to 2.000 V (vs. Li/Li+) at room temperature, using graphite as working electrode, Li as reference and counter electrode at a scanning rate of 0.2 mV s-1.
3. Results and Discussion
3.1 Effect of BATE with different concentrations on the cell cyclability
The cycle performances of the graphite anode in EC-based electrolytes with different concentrations of BATE were presented in Figure 1. The graphite electrode exhibited highest cycle capacity and cycle stability for the electrolyte which contains 1.0% BATE, and the capacity retention ratio was improved by adding 2.0% BATE in the EC-based blank electrolyte, but the capacity decreased. For the blank electrolyte without BATE addition, the capacity faded rapidly. These results indicated that the addition of BATE in the EC-based electrolyte significantly enhanced the cycle performance of the graphite electrode, and BATE was feasible for use as a film-forming additive to improve the performance of commercial lithium-ion batteries. The case was similar to tris(trimethylsilyl) borate (TMSB) as an additive in EC-based electrolyte [13]. Electrolyte containing 0.5% BATE showed high capacity during the first several cycles, nevertheless, its capacity retention ratio was not high as expected. This may be related to SEI film did not fully form on the graphite anode. Based on the above results, the addition of BATE to electrolyte should be controlled.
3.2 Effect of BATE with different concentrations on the cell cycling efficiency
Figure 2 showed the cycling efficiency of Li/graphite cell in EC-based electrolytes with different concentrations of BATE. For organic electrolyte additives, electrochemical decomposition or cointercalation with graphite anode frequently happened during chargedischarge cycle. If a uniform SEI layer could not form, columbic efficiency of cycling would be low [14]. However, we found that BATE was quite compatible with graphite anode. The initial efficiency with addition of BATE was comparable to that with blank electrolyte, and the charge-discharge efficiency increased after the second cycle. As shown in Figure 2, the cycling efficiency with BATE was higher than that with blank electrolyte, and it increased as BATE concentration increased.
3.3 Effect of BATE with different concentrations on the cell impedance
During cycling an internal impedance of cell increased. And that would cause capacity fading. In addition to the charge-discharge test, impedance measurements were also performed during different cycle, the Nyquist plots were presented in Figure 3. The impedance plots of Li/graphite cells with different electrolytes (Figure 3a) exhibited similar impedance behaviors: a semicircle at high frequencies reflected the interfacial impedance of SEI and charge-transfer resistances, and a slop line at low frequencies was assigned as the Warburg impedance which was associated with the ions diffusion process in the electrode materials. As the charge-transfer resistance is related to the semicircle diameter, the smaller the semicircle diameter, the smaller the charge transfer resistance [15,16]. Impedance of cell with 2.0% BATE was much higher than that with other concentrations, maybe due to high viscosity of electrolyte with too much BATE or the formation of a too thick and compact SEI film, which increased ion transfer resistance and limited the capacity utilization. Balakrishnan et al. [17] supposed that the deterioration of cell performance is owing to either electrochemical instability or additive viscosity increase (which leads to capacity fading or affects capacity utilization). The impedance plots of Li/graphite cells after 20 cycles were shown in Figure 3(b), the impedance of all cells increased with cycling. Whereas, the increase of cell with blank electrolyte and with 0.5% BATE changed significantly. This could be related to electrochemical instability for lack of a uniform SEI film formed during the first charge-discharge process.
3.4 Effect of BATE with different concentrations on the cell cyclic voltammetry
As an SEI-forming additive, the preferable reduction to electrolyte solvents is important [18]. The cyclic voltammetry curves of the Li/ graphite cells with different electrolytes were displayed in Figure 4. From the partial enlarged view (inset a), obvious irreversible peaks at 1.6 V-1.9 V (vs. Li/Li+) were observed only in the electrolytes containing BATE. These peaks were related to the BATE reduction. From the partial enlarged view (inset b), a strong reductive irreversible peak at 0.5 V-0.8 V (vs. Li/Li+) in BATE-free electrolyte was observed obviously. The reductive peak was attributed to the decomposition of EC molecules and associated with the formation of SEI film on the graphite electrode surface. Peak of cell in the electrolyte with BATE disappeared at 0.5 V-0.8 V (vs. Li/Li+), which meant that BATE could mitigate EC decomposition at approximately 0.6 V and increase the electrolyte durability.
Current density of the reversible peak barely decreased during cycle, which implied the good cycleability of graphite electrode in the electrolyte [19]. The cyclic voltammetry curves shown in Figure 5 present the change of reversible peak current density before and after charge-discharge test. Compared with the curves of cells with different electrolytes in Figure 5(a) and 5(b), current density of the reversible peak for blank electrolyte showed a great decrease, so it is the same case for 0.5% BATE electrolyte. However, current density of the reversible peak were relatively stable in electrolytes with 1.0% and 2.0% BATE respectively, which also indicated the good cycleability in the charge-discharge test.
3.5 Effect of BATE with different concentrations on graphite anode morphology
Figure 6 presented SEM micrographs of the graphite electrode surface before and after cycled in different electrolytes. Before cycling, the graphite particles with a little adhesive/conductive agent could be identified in the pristine electrode as shown in Figure 6(a). After 20 cycles, the anode in blank electrolyte without BATE was covered by a film. So the graphite particles could not be observed any more, of which the cell exhibited a faster capacity fading in the chargedischarge test, as shown in Figure 6(b). At the graphite anode, surface fractures in graphite particles can be induced with cycles, the subsequent surface area increase and electrolyte reduction reactions that consume more cycleable Li [20,21]. The morphology of the graphite electrode after 20 cycles with 0.5% BATE electrolyte was similar to the blank electrolyte (Figure 6(c)), which acted the same behavior in the cycling test as cell with blank electrolyte, owing to the incomplete SEI film formed by small BATE. Morphologies of electrodes with 1.0% and 2.0% BATE electrolyte were uniform, and the surfaces of the electrodes were covered with compact film (Figure 6(d) and (e)), which indicated that a well stable SEI was formed [22]. It seemed that a thick SEI film could prevent the graphite anode from direct contacting with the electrolyte more adequately. However, a too thick and compact film can deteriorate the cell performance because it may retard Li+ transfer and decrease the capacity performance [23]. So amount of addition of additive to electrolyte should be controlled.
4. Conclusion
In this study, the compound of BATE, a glycerol carbonate derivative, added in 1mol L-1 LiPF6-EC:DMC (1:1, v:v) as electrolyte additive was investigated by charge-discharge test, electrochemical impedance test, cyclic voltammetry test and scanning electron microscope test. As an electrolyte additive, BATE obviously improved cycle performance of the cell. Especially cell with 1.0% BATE electrolyte had highest capacity and highest capacity retention in long cycles, and it had higher cycling efficiency than cell with blank electrolyte. The improvements attributed to a stable and protective SEI film with a low resistance on the anode surface formed by BATE reduction prior to electrolyte solvents. The SEI film could suppress electrolyte decomposition and prevent graphite electrode from destruction effectively.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
All the authors substantially contributed to the study conception and design as well as the acquisition and interpretation of the data and drafting the manuscript.