1. Introduction
The development of the structural materials in the automotive, electronic and aerospace applications promotes the use of magnesium and its alloys [1] due to many benefit such as high specific strength and energy absorption and low density [2]. Unfortunately, there are still many important questions to be considered for their wider applications in both fundamental and technology. The current focuses of magnesium alloy technology were suggested by Agnewa and Nie [3]. They reported the trends which is a perspective for future research to innovate the alloy properties and extend magnesium alloys’ applications. But, the major concern of magnesium and its alloy is poor corrosion resistance [4]. For this reason, the improved corrosion resistance for magnesium alloys has become an important issue to be dealt in the corrosion research field and must be improved to achieve the better corrosion resistance required for their applications. The heavy metals including iron, nickel and copper… were considered as alloying element for magnesium alloys. However, they are highly deleterious because of low hydrogen overvoltage. The aluminum-iron or nickel-magnesium compounds which are formed by iron or nickel impurities, act as local cathodes to form microgalvanic corrosion [5,6]. This requires more active elements to be used as alloying element with reduction potentials closed that of Mg including Al [7,8], Ca [9-11], Sr [12-15] and refines the microstructure by the rapid solidification process [16-19] improved the corrosion resistance. The beneficial effects which they passes to increase in the corrosion resistance of magnesium alloys can be attributed to: (i) decrease in the impurities, (ii) prevention of micro-galvanic couples, (iii) formation of perfect precipitation around finer α-Mg grains, and (iv) ameliorate the surface film [20].
The aluminum addition seems to be the most commonly used due to good castability and corrosion resistance as well as good mechanical properties [20]. Furthermore, Mg17Al12 is better corrosion-resistant than α-Mg matrix because it can act as a corrosion barrier [21]. However, when magnesium alloyed with aluminum, it becomes electrochemically more active [23] due to nonuniform Al distribution. Thus, larger amount of aluminum content is referred to an improved corrosion resistance of Mg alloy. Therefore, the effect of a small amount of aluminum (not exceed 5 wt.%) in the Mg alloy is still questionable in case of corrosion resistance. The previous studies [23- 29] suggest that the corrosion properties of magnesium alloys depend on the amount of intermetallic phases and inclusions as well as their distribution in the alloy. They act as a corrosion barrier once the less noble α phase was dissolved. β(Mg17Al12) phase have a passive behavior within a wide pH range resulting in an improved corrosion resistance. However, it also has deleterious influence on corrosion resistance due to microgalvanic corrosion effect [30-31]. Theoretically, impurity elements form microgalvanic corrosion attributed to an effective cathode material in the galvanically couple, or if the nature of the environment inhibits formation or maintenance of a protective film [33,34]. That is the reason why magnesium alloys is poor quality. In addition, the electrical contact between the magnesium alloys and other metals can be insulated or blocked, resulting in reduction in microgalvanic corrosion.
Tin is believed to be an innocuous background material with many applications in the metallurgy industry due to its corrosion resistance. House and Kelsall [35] reported that tin reacts to produce hydrogen in both strongly acidic and alkaline solutions, even though the stability of the valence +2 state is considerably higher. The oxidation of Sn (II) to Sn (IV) proceeds easily in aqueous solutions due to the dissolved oxygen, making Sn (IV) stable in aqueous solutions [36]. An outer SnO2 layer could be formed on alloy surface for corrosion protection. In addition, Song [37] has reported that the role of tin is to improve the corrosion resistance of magnesium alloys immersed in a corrosive medium. A beneficial effect on corrosion behavior of magnesium alloys can be attributed to the presence of Sn-containing particles and the solute Sn in the matrix phase. It can change the electrochemical anodic and cathodic polarization behavior of the alloy. Therefore, Sn has been strongly considered as an alloying element to improve the corrosion behavior of magnesium alloys. Herein, the study examined the alloying effect of tin on the corrosion behaviour of Mg-5Al based alloy. In order to determine the corrosion behavior of Mg-Al alloys in corroded solutions, Mg-5Al-xSn (x = 0 ÷ 4), alloys are studied based on the basics of electrochemical measurements. The surface products were analyzed by X-ray photoelectron spectroscopy (XPS).
2. Experimental Procedure
2.1 Specimen preparation
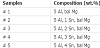
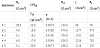
Pure Mg (99.9 %) ingot was melted in a stainless steel crucible under the protection of gas mixture containing SF6 and CO2. The calculated amounts of 5 wt.% of Al: 1, 2, 3, and 4 wt.% Sn were added to Mg melt. After solidification, the ingots were subjected to homogenizing treatment at 400°C for 14 h. The homogenized ingots were machined, which were used as raw materials for extrusion. The extrusion of billets was performed at 320°C. Table 1 summarizes the alloy designation and the corresponding chemical composition of each alloy. The chemical compositions of tested alloys were determined by means of elaboration method. Alloys with chemical compositions used, were 0.010 Mn, 0.005 Si, 0.004 Fe, 0.003 Cu, 0.007 Ni, while the difference between measured and specified composition of tin and aluminum is imperceptible. The specimens for electrochemical tests were first cold-mounted on a mounting cup and then finished by grinding with 600-grit silicon carbide (SiC) paper.
2.2 Electrochemical investigation methods
All of the electrochemical experiments were performed at room temperature in 3.5 wt.% NaCl solution. The exposed area was 1 cm2. Potentiodynamic polarization tests were performed using an EG&G PAR 263A potentiostat for the DC measurements. A graphite counter electrode was used, with a saturated calomel electrode as the reference electrode. Prior to the potentiodynamic polarization test, the samples were kept in the solution for 1 h in order to stabilize the open-circuit potential. The potential of the electrodes was swept at a rate of 0.166 mV/s in the range from the initial potential of -250 mV versus Ecorr to the final potential of -1.3VSCE. The electrochemical impedance spectroscopy (EIS) and corrosion potential measurements were conducted using a IM6e system with a commercial software program for AC measurements. The amplitude of the sinusoidal perturbation was 10 mV. The frequency range was from 100 kHz to 10 mHz. The hydrogen evolution rate of the alloys was investigated by immersion tests. The specimens, with dimensions of 10 mm × 10 mm × 2 mm, were prepared by grinding each side with 600-grit silicon carbide (SiC) paper and degreasing the surfaces with ethanol prior to corrosion testing. The hydrogen evolution rate was used as an indicator of the corrosion rate which was monitored after 1 hour.
2.3 Surface analyses
The crystal structure of the Mg-5Al-xSn specimens was investigated by XRD using Cu Kα radiation. For the observation of the microstructure using optical microscope, the specimens were mechanically sanded with sand paper (#220, 600, 1200, 2000, and 4000) and then with 0.1 μm alumina powders. These specimens were then etched in a mixture of acetic acid (10 ml), picric acid (5 g), distilled water (10 ml) and ethanol (70 ml of 95% purity). To investigate the relationship between the electrochemical behavior and surface morphology, the specimens were examined by scanning electron microscopy (SEM) after 6 h of immersion test. The surface products were examined by X-ray photoelectron spectroscopy (XPS) after 1 h of the open-circuit potential.
3. Results and Discussion
3.1 Alloy microstructure
Microstructures of Mg-5Al-xSn alloys with different Sn-containing alloys are shown in Figure 1. It showed the typical microstructure of an alloy and there were no second phases such as Mg17Al12 and Mg- Zn compounds located at the grain boundary. It could be attributed to the dissolution of those compounds into the α-Mg matrix. It can be noted that the grain size distributions were homogeneous due to the homogenizing treatment at 400°C prior to 14 h. It also can be seen that a large amount of new fine grains appeared at the grain boundary in the Sn-containing specimens due to recrystallization. The grain size decreased with an increasing of Sn addition.
Figure 2 shows the XRD pattern of Mg-5Al-xSn alloys. All specimens have the defined peaks of Mg and Mg12Al17 reflections, new peaks are also detected in the case of Sn-containing specimens. The new peaks are close to the reflections of Mg2Sn. Importantly, the peak positions of Mg2Sn coincides well with those of standard XRD patterns, suggesting that the main second phase in the homogenized condition is Mg2Sn. It was also noted that the intensities of the Mg2Sn phases increase with increasing of Sn content, implying that the solubility of Sn in Mg varies with the Sn content in Mg-5Al alloy. In addition, no Mg12Al17 and Mg2Sn particles are present at the grain boundaries, indicating that they were completely dissolved into the α-Mg matrix during the heat-treatment.
3.2 Electrochemical properties
Figure 3 shows the polarization curves of the Mg-5Al alloys as a function of the Sn content in 3.5 wt.% NaCl solution. The results showed that all specimens exhibited only active behavior where the anodic current increased with increasing potential, indicating the absence of a passive film on the specimen surface. The potentiodynamic polarization tests indicated that the addition of Sn from 0 to 3 wt.% decreased the corrosion current density. Further additions of Sn from 3 to 4 wt.% Sn increased the corrosion current density considerably. Table 2 lists the corrosion properties.
Figures 4a and Figure 4b present the Nyquist and Bode plots after immersion for 1 h at Ecorr. The high spectra are used to detect the local surface defects, whereas the medium and low frequency spectra detect the processes within the corrosion product and at the metal/corrosion product interface, respectively. The impedance spectrum of the Mg- 5Al-xSn alloy exhibits a capacitive loop in the high frequency range and an inductive loop in the low frequency range. The aperture of impedances and impedance modulus |Z| of the Sn-containing alloys are greater than that of the Mg-5Al alloy. The measured spectra were fitted by the ZSimpWin program with a defined equivalent circuit in Figure 5 a. Where, Rs is the solution resistance, CPEdl is double layer capacitance and Rct is the charge transfer resistance of the magnesium alloys, L and RL are the inductance and resistance which represented the breakdown of partial protective film on alloy surface [38]. In this case, the capacitor was replaced with a CPE to improve the fitting quality, where the CPE includes a double-layer capacitance (C) and phenomenological coefficient (n). The CPE is a useful modeling element with impedance given by the following equation [39]:
where Q0 is the admittance that is equal to the inverse of the impedance (Z) at ω = 1 rad/s, j is an imaginary number and n is the CPE power. The n value of a CPE indicates its meaning: n = 1, capacitance; n = 0.5, Warburg impedance; n = 0, resistance and n = -1, inductance. The polarization resistance, Rp, can be calculated from the equivalent circuit as shown below:
Figure 5b shows fitting results for experimental impedance spectra of the Mg-5Al-xSn alloys in 3.5 wt.% NaCl solution. The quality of the fitting to the equivalent circuit is judged on the basis of the % error and Chi-square (χ2) value. Fitting results showed that the % error and χ2 value were in agreement, suggesting the development of processes are suitable for the defined equivalent circuit. The fitting results are presented in Table 2. It indicates that resistances of Sn-containing specimens are much higher than that of Mg-5Al based alloy.
Hydrogen evolution rate of the Mg-5Al-xSn alloys immersed in 3.5 wt.% NaCl is shown in Figure 6 a. It showed that in a 7 h immersion, hydrogen evolution rate of the Mg-5Al-xSn decreased in the order: Mg-5Al > Mg-5Al-4Sn > Mg-5Al-1Sn > Mg-5Al-2Sn > Mg-5Al-3Sn. This means that the Mg-5Al-3Sn alloy exhibited the best corrosion resistance, and the Mg-5Al had the lowest corrosion resistance. Corrosion rate of the Mg-5Al-xSn alloys were calculated based on the emitted hydrogen volume and presented in Figure 6b. It indicated that the corrosion rate of the Sn-containing samples increased slightly during 7 h, while it increased quickly in case of Mg-5Al based alloy. The lower corrosion rate could be attributed to the formation of a protective corrosion product layer.
Figure 7 shows the change in corrosion rates of the Mg-5Al-xSn alloys. This suggests that the addition of Sn improves the corrosion properties of Mg-5Al based alloy. Average corrosion rate for Mg- 5Al-xSn alloys obtained by hydrogen evolution and electrochemical methods are compared in Figure 7. The corrosion rate can be confirmed by the corrosion current density according to Faraday’s law and hydrogen evolution rate. The corrosion rate of Mg-5Al-xSn alloys in the electrochemical tests was calculated by Faraday’s law [40,41]:
where 3.16 x 107 is the metric and time conversion factor, icorr is the corrosion current density (A/cm2), M is the molar mass of the metal (g/mol), z is number of electron transferred per metal atom, F is the Faraday’s constant, and ρ is the density of the metal (g/cm3). Polarization resistance (Rp) value was obtained from EIS data according to eq. (4) [41]:
where βa and βc are the anodic and cathodic Tafel slope, respectively. The corrosion current density of the alloys was calculated using Equation 4 under the assumption that βa and βc were equal 0.1 V/ decade. Similarly, corrosion rate of the alloys was calculated from the the EIS measurements by using Equation 3 [42-44]. In addition, the hydrogen evolution volume rate, VH (ml/cm2.d) can be related to the corrosion rate as, PH (cm/y) = 0.2279 VH [45-47]. The results suggested that Sn-containing alloys have lower corrosion rates than Mg-5Al based alloy. Hence, the electrochemical and hydrogen evolution measurements indicate that the corrosion behaviour of Mg-5Al alloy was improved by Sn addition. This improvement could be related to the formation of a protective film on the alloys.
3.2 Surface analyses
Figure 8 shows SEM images of the surface morphology after 7 hours immersion. There was a significant difference in the surface morphology of the alloys, due to the pitting, which was inwardly penetrated of Cl¯ ion. No pitting was observed on the surface of the Mg-5Al-3Sn alloy, whereas there was pitting corrosion on the Mg- 5Al, Mg-5Al-1Sn, Mg-5Al-2Sn, and Mg-5Al-4Sn specimens.
X-ray photoelectron spectroscopy was performed to characterize the changes after exposing the alloys to the 3.5 wt.% NaCl solution for 1 h at room temperature. Figure 9 shows the XPS full range survey spectra of the samples, which clearly showed peaks for Mg 2s, Al 2p, Sn 3d, Cl 2p, and O 1s, indicating the existence of Mg, Al, Sn, Cl, and O on the specimen surface. Oxygen KLL [48,49] was also detected on the surface. Figure 9 (b-f) shows the XPS spectra of Mg 2s, Al 2p, Sn 3d, Cl 2p, and O 1s on the alloy surfaces. Mg 2s was observed on the surface of the Sn-containing alloys, whereas there was no Mg 2s present on the Mg-5Al based alloy. The Mg 2p and Al 2p spectra correspond to MgO/Mg(OH)2 and Al2O3/Al(OH)3 on the surface of the alloys. This figure also shows that the concentration of Al 2p and O 1s increased with increasing Sn content of 3 wt.% Sn, and decreased with further increase in Sn content. The binding energy of Sn 3d3/2 and Sn 3d5/2 was approximately 494 and 485 eV, respectively Figure 9d. The presence of SnO2 on the surface of the Sn-containing alloys decreased the corrosion rate due to a decrease in the hydrogen evolution rate. Compared to the Sn 3d oxide peaks in Figure 8d, the alloy contribution for the 3 wt.% Sn-containing alloy has higher peaks than those peaks from other alloys. In addition, the O 1s spectra were composed of three peaks corresponding to the signals from oxygen in the oxide at 530.05 eV and oxygen in the hydroxyl groups at 531.70 eV. In addition, a lower Cl peak was observed in the Mg-5Al-3Sn specimen. This suggests that the enriched Mg, Al, and Sn products containing played an important role in improving the corrosion products of magnesium alloys, which impeded the adsorption of Clions.
4. Discussion
Five different tin-containing Mg-5Al alloys, 0, 1, 2, 3, and 4 wt.% additions, were studied. The microstructural study including OM and XRD suggests that a decrease in grain size of the α-Mg solid solution phase was the major change in the microstructure caused by the addition of Sn. The electrochemical study suggests that the addition of 3 wt.% Sn results in the lowest corrosion rate and hydrogen evolution rate in a corrosive environment. The corrosion resistance mechanism of the alloys can be related to the finer α-Mg grains and no Mg12Al17 and Mg2Sn particles remaining at the grain boundaries, which strongly affect the corrosion performance through control of the microstructure. Therefore, a higher level of total resistance, lower corrosion rate and hydrogen evolution reaction rate were obtained with increasing Sn addition to Mg-5Al alloy in the range, 0 to 3 wt.%, as shown in Table 3 and Figures 4, 6, and 7. Corrosion of Mg can convert metallic Mg to the stable ion Mg++. The overall corrosion reaction is:
Mg + 2H2O → Mg(OH)2 + H2
The reaction (5) is expressed as the sum of the following reactions:
Mg → Mg2+ + 2e-
2H2O + 2e- → 2OH- + H2
Mg2+ + 2OH- → Mg(OH)2
Furthermore, magnesium oxide (MgO) was observed due to the following reduction reactions:
Mg2++ H2O → MgO + 2H+
Therefore, magnesium oxides/hydroxides (MgO/Mg(OH)2) should form on the surface of magnesium upon its exposure to the corrosive environment [50]. Based on the Pourbaix diagram of the Sn/H2O system [35] and the surface characteristics of the Sn-containing alloys in 3.5 wt.% NaCl solution, the formation of SnO2 in this environment was proposed to proceed through the following reaction scheme:
Sn + 4H2O → Sn(OH)4 + 4e- + 4H+
Sn may also be oxidized according to the following reactions:
Sn + 2H2O → Sn(OH)2 + 2e- + 2H+
Sn(OH)2 + 2H2O → Sn(OH)4 + 2e- + 2H+
The following dehydration reaction can take place [51]:
Sn(OH)4 → SnO2 + 2H2O
Therefore, when Sn was added to the alloy, the Sn oxide was enriched in the indicated content range due to the occurrence of Sn oxides through the formation of a corrosion product layer, as shown in Figure 9d. The surface characteristics revealed SnO2 to be the main oxide. Therefore, the difference in total resistance and hydrogen evolution rate is caused by a difference in the composition and quantity of surface products formed on the alloy surfaces. However, total resistance increased and hydrogen evolution rate decreased strongly with increasing Sn content from 0 to 3 wt.% Sn due to surface film formation, and decreased with further increases in Sn content. The corrosion products formed is certainly dense enough to protect the alloy surface during the lengthy immersion time. For 0 ÷ 3 wt.% Sn specimens, the protection layer that formed in the initial stage of the alloy surface can prevent the metal from being corroded further. Grain refinement can increase the number of active atoms on the surface, accelerating the formation of the protective layer. Therefore, Sn addition (0 ÷ 3 wt.%) with grain refinement has an increase in the corrosion resistance of Mg-5Al alloy. This results in the formation of an oxide protection layer. This is attributed to the uniform distribution of Sn in the α-Mg matrix, and is also related to the uniform distribution of Sn oxides on the growth of corrosion products. There is a general hindrance of corrosion from the fresh surface of Sn oxides. This might be related to the observation that grain refinement and sufficient second phase have a lower tendency for localized grain boundary corrosion, whereas in higher second phase (Mg2Sn) containing specimen, this can result in failure when pitting effect arises, as shown in Figure 8e. It can begin as local galvanic attack relating to the Mg2Sn phase distribution and Mg. In addition, the polarization may reduce the potential difference of the couple as the galvanic current develops. These accelerate the combination of atomic H on surfaces to form the hydrogen gas that evolves. Therefore, the decrease in the corrosion resistance of Mg-5Al-4Sn was performed. This is coincident with the results shown in the electrochemical and surface analyses. In summary, the results suggest that the corrosion behaviour of Mg-5Al alloy can be improved by the addition up to 3 wt.% Sn. On the other hand, the addition of 4 wt.% Sn accelerates the corrosion rate of Mg- 5Al alloy. Clearly, the presence of beneficial Sn compounds on the outer layer of the protective film inhibits the ability of chloride ion to penetrate and destroy the alloy. The beneficial Sn compounds might concentrate the protection of the corrosion product layers. In the case of 0 ÷ 3 wt.% Sn alloy, the Sn concentration was sufficient to act as a barrier to Mg-5Al alloy dissolution, which enhances the corrosion properties of alloy. On the other hand, the higher Sn content in the 4 Sn alloy had a higher corrosion rate.
5. Conclusion
The major changes in the microstructure caused by the addition of Sn were the decrease in grain size of the α-Mg phase and increase of Mg2Sn phase in the Mg matrix. The corrosion rate decreased with increasing Sn. However, the addition of 4 wt.% Sn did not improve the corrosion resistance due to acceleration of the hydrogen evolution reaction rate. The corrosion products on the surface of the Sn specimens added from 0 to 3 wt.%, which was composed mainly of SnO, could improve the corrosion resistance of the Mg-5Al alloys in 3.5 wt.% NaCl solution.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
Author Contributions
All the authors substantially contributed to the study conception and design as well as the acquisition and interpretation of the data and drafting the manuscript.
Acknowledgments
The authors are grateful for the support of Vietnam Oil & Gas Group, PetroVietnam University.