Hypertension, hyperlipidemia, diabetes, and obesity are the "deadly quartet" of lifestyle related diseases, those whose onset and slow progression are the result of choices made in everyday living. Each condition is bad enough alone, but combined they significantly increase the risk of death. Atherosclerotic lesions are a frequent hallmark of lifestyle-related disease; they develop silently and often go undetected until their condition and location precipitate myocardial and/or cerebral infarction and, not infrequently, sudden death.
Atherosclerosis and its complications are among the leading causes of mortality in Western countries [1] and a similar trend is reported in Japan [2]. Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in both adult and pediatric populations [3,4]. Clinically it is divided into simple fatty liver without inflammation and fibrosis and steatohepatitis; if left untreated, however, progression to steatohepatitis is nearly certain. Severe NAFLD due to diabetes mellitus and obesity commonly advances to inflammatory non-alcoholic steatohepatitis (NASH), a precursor of cirrhosis and ultimately hepatocellular carcinoma [6]. Since NAFLD and atherosclerosis share common molecular mediators, NAFLD itself might play a crucial role in the development and progression of atherosclerosis [5].
Animal studies are crucial to the development of therapeutic drugs for atherosclerosis and NAFLD. A gene-targeted mouse model of atherosclerosis has been created at the Jackson Laboratory in Bar Harbor, Maine, USA, in which C57BL/6J mice homozygous for the ApoEtm1Unc mutation demonstrate a functional knockout of the antiatherogenic apolipoprotein E (ApoE) gene involved in cholesterol metabolism [7,8]. This ApoE-knockout (ApoE-KO) mouse has been widely used in atherosclerosis research because of its propensity to spontaneously develop hypercholesterolemia and atherosclerotic lesions similar to those found in humans [9,10]. In addition to atherosclerotic lesions, ApoE-KO mice over 6-months of age develop hepatic steatosis and fibrosis [11].
Atherosclerotic lesions in ApoE-KO mice are histopathologically classified into 3 categories by stages [12]: i) early, ii) progressive/ advanced, and iii) combined lesions. Early lesions are simple accumulations of foamy cells in aortic endothelial epithelium (Figure 1A). Progressive lesions are composed of foamy cell accumulations, fibrous caps and lipid cores (Figure 1B). Combined lesions show accompanying calcification and/or ossification (Figure 1C), with occlusion of the lumen in progressive lesions.
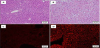
Histopathological findings in ApoE-KO livers are presented in Figure 2. Compared to normal liver cells in wild-type mice (Figure 2A), variable hepatocyte size with cytoplasmic vacuolization are evident without the presence of inflammatory cells and fibrosis (Figure 2B). Frozen liver sections stained with Nile Red for demonstration of lipids showed that, while normal liver shows almost no signals (Figure 2C), ApoE-KO livers demonstrate numerous lipid droplets within hepatocytes (Figure 2D), indicating fatty metamorphosis similar to simple fatty liver in human NAFLD.
In a practical sense, mice are the preferred research animal in terms of economical space and husbandry requirements, and the ability to generate specific gene equivalency between mouse and man is extremely useful in translational research. Because both atherosclerosis and NAFLD normally occur in this line and are promoted by dietary fat, ApoE-KO mice provide a functional model to aid in the elucidation of molecular mechanisms and the development of therapeutic agents for treatment of human atherosclerosis and NAFLD.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.