1. Introduction
Simple hepatic cysts are benign cystic formations that occur frequently in the general population, with a prevalence ranging from 2% to 4% [1-3]. Most benign, nonparasitic hepatic cysts are found incidentally and are usually asymptomatic. However, complications such as obstructive jaundice [4], rupture [5-9], intracystic hemorrhage [9-15], and infection [16-24] can occasionally occur. Most patients with a hemorrhagic hepatic cyst present with abdominal pain (80%), while some cases are asymptomatic at the time of presentation [15].
Malignant neoplasms occasionally present in damaged liver. Intracystic hemorrhage and cystadenocarcinoma of the liver sometimes cannot be reliably differentiated on imaging studies. Even when diagnosed accurately, the optimal treatment strategy remains controversial. Hepatectomy is usually performed in this situation [9,11].
We describe a patient who had an asymptomatic hemorrhagic hepatic cyst with solid contents that mimicked a malignancy.
2. Case Report
An 85-year-old woman was referred to our hospital because a hypoechoic mass was detected in the liver on physical examination. The patient had no complications. Her past medical history included hypertension and a left femoral-artery aneurysm. She did not have a bleeding tendency and was not receiving antiplatelet agents or anticoagulant agents.
The results of initial laboratory tests were as follows: serum aspartate aminotransferase, 28 IU/L (normal, <28 IU/L); serum alanine aminotransferase, 23 IU/L (normal, <33 IU/L); serum lactic dehydrogenase, 238 IU/L (normal, 180 to 460 IU/L); serum gamma glutamic transpeptidase, 20 IU/L (normal, 8 to 39 IU/L); serum albumin, 4.0 g/dL (normal, 3.8 to 5.3 g/dL); serum C-reactive protein, 0.11 mg/dL (normal, <0.3 mg/dL); white blood cell count, 5600/µL (normal, 4000 to 8000/µL); red blood cell count, 407×104/µL (normal, 410 to 550×104/µL); and serum hemoglobin concentration, 13.1 g/ dL (normal, 14 to 18 g/dL). The serum platelet count was 17.3×104/ µL (normal, 20 to 40×104/µL) and was thus slightly decreased. The prothrombin time was 127.6% (normal, 70% to 130%), and the activated partial thromboplastin time was 26.0 s (normal, 24 to 37 s). The serum concentration of carcinoembryonic antigen was 3.0 ng/mL (normal <2.5 ng/mL), that of CA19-9 was 12.2 u/ml (normal <37), and that of AFP was 3.8 ng/ml (normal <10). The serum concentration of hyaluronic acid (147 ng/mL) was elevated (normal <50 ng/mL). Hepatitis B virus antigen and hepatitis C virus antibody were negative.
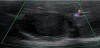
Ultrasonography revealed a protruding hypoechoic mass, 6 cm in diameter, with a central isoechoic structure in segment 7, accompanied by multiple hepatic cysts. On Doppler ultrasonography, no blood flow was detected in the hypoechoic mass. The edge of the liver was dull, and the liver parenchymal echo was mildly rough (Figure 1).
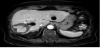
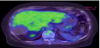
Computed tomography (CT) showed a slightly high-density area with calcification of the margin as compared with that of the other hepatic cysts (Figure 2). Magnetic resonance imaging (MRI) revealed a hypointense lesion with central hyperintensity on T1-weighted sequences and hyperintensity on T2-weighted sequences (Figure 3). On positron emission tomography (PET)-CT, there was no uptake of fluorodeoxyglucose (FDG) in the liver (Figure 4).
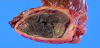
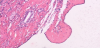
We diagnosed an old hemorrhagic cyst or abscess, but could not rule out a malignant neoplasm because of the damaged liver. After obtaining informed consent, a laparotomy was performed. The mass protruded from the liver, and adhesion to right diaphragm was detected (Figure 5). A right lateral sectionectomy was performed. The resected specimen had black solid contents, and the capsule fluid (Figure 6). Pathological examination revealed an old hemorrhage with a thick fibrous capsule without epithelial components. The liver showed mild fibrosis with fatty changes. The mass was considered an old hemorrhagic hepatic cyst because a nearby hepatic cyst had simple columnar epithelium (Figure 7). The postoperative course was uneventful, and the patient was discharged 8 days after operation.
3. Discussion
Simple hepatic cysts can be congenital or acquired and occur more frequently in females and elderly patients. They are common and benign, usually asymptomatic, and require no treatment [15,25]. They occasionally resolve spontaneously, but can also cause serious complications [4-14,16-23,26]. Fong et al. reviewed the literature on hemorrhagic hepatic cysts [27]. The mean age was 62.7 years, and the mean size of the cyst was 11.2 cm. Most patients with hemorrhagic cysts presented with abdominal pain (80%), while 14% of the patients were asymptomatic at presentation. Treatment recommendations for symptomatic hepatic cysts include surgery [28-30] and the injection of a sclerosing agent into the cyst [31-33]. The clinical presentation of spontaneous intracystic hemorrhage usually begins with severe abdominal pain of sudden onset, followed by a gradual decline in pain and healing in response to conservative therapy [10,12,13,34]. In our patient, the cyst was asymptomatic at the time of presentation. However, Marion et al. [35] reported a case of hemorrhagic hepatic cyst rupture that presented with hemorrhagic shock.
In most cases, the causes of intracystic hemorrhage are unclear. The wall of a hepatic cyst consists of three layers: an inner layer of loose connective tissue lined with cylindrical or cuboidal epithelium, a middle layer of compact connective tissue containing blood vessels, and an outer layer of loose connective tissue with large blood vessels, bile ducts, and occasional von Meyenburg complexes. On exposure to high intracystic pressure, the epithelial lining may undergo necrosis and sloughing. In our patient, the presence of calcification of the cystic wall suggested that sclerosis of the blood vessels in the cyst wall may have caused the intracystic hemorrhage [36]. Hemorrhage might occur repeatedly, gradually contributing to the size of the cyst.
On ultrasonography, hemorrhagic hepatic cysts typically contain fluid that is hyperechogenic as compared with that in simple cysts. On MRI, intracystic hemorrhage is frequently associated with internal signal intensities. Hyperintensity on T1- and T2-weighted MRI sequences can help to differentiate intracystic hemorrhage from other cystic lesions. On T1-weighted images, the signal intensity of fluid changes from low to high as the protein concentration increases [37]. In our patient, the hemorrhagic cyst was a hypointense mass with central hyperintensity on T1-weighted sequences and hyperintensity on T2-weighted sequences. The signal intensity of hemorrhage decreases when clots are liquefied [38]. Fong et al. reported imaging workup data obtained in 15 patients with hemorrhagic hepatic cysts who underwent a combination of abdominal ultrasonography, CT, and MRI. On T1- and T2-weighted MRI of the abdomen, eight patients (53.3%) showed hyperintense signals and the other seven (46.7%) had a mixture of hyperintense and hypointense signals. Of the seven patients with mixed signals, three showed intracystic hyperintense signals surrounded by a rim of hypointensity. The other four patients exhibited hyperintensity with scattered hypointense nodularity [15]. Calcification of the cyst wall may occur, but is also seen in nonhemorrhagic simple cysts. Most cyst walls are thin and smooth and are not enhanced on CT or MRI after intravenous injection of contrast medium. However, enhanced thick walls are occasionally seen in the presence of inflammation, granulation, or fibrosis. After hemorrhage, cyst fluid is usually hyperdense on CT, hyperintense on T1-weighted MR images, and hypointense to hyperintense on T2-weighted images. A fluid–fluid level is sometimes observed.
Differentiating hepatobiliary cystic neoplasms from simple hepatic cysts complicated by intracystic hemorrhage can be difficult because both lesions have intracystic structures [13,39]. Cytologic examination of cystic fluid obtained by aspiration may provide important information for distinguishing malignant from benign lesions [11]. However, aspiration of a cystic lesion can lead to tumor spread.
We diagnosed an old hemorrhagic cyst or abscess, but could not rule out a malignant neoplasm because of liver damage. A sectionectomy was therefore performed. On macroscopic examination, the resected specimen had black solid contents with a capsule that lacked fluid. Pathological examination revealed an old hemorrhage with a thick fibrous capsule without epithelial components. The mass was considered an old hemorrhagic hepatic cyst because a nearby hepatic cyst had simple columnar epithelium.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
All authors contributed substantially to conception and design, acquisition and analysis of data and interpretation of results.