1. Introduction
Diabetes Mellitus (DM) or shortly diabetes is used as the collective name of several chronic metabolic diseases of the human body where by impaired insulin production the human body cannot automatically regulate the glucose level [1,2]. Different types of DM exist associated with diverse symptoms and side effects. The main types are the following [3]:
- Type 1 or insulin-dependent DM (T1DM);
- Type 2 or insulin-independent DM (T2DM);
- Gestational DM (GDM);
- Double DM (DDM);
- Genetic related DM (GrDM);
- Other types of DM which can be induced by different kind of diseases, drugs, chemicals and cancers [3].
Type 1 and type 2 cover more than 95% of the cases, T1DM being more critical. T1DM is an autoimmune disease, which mainly appears in young age and its general medical symptom can be described by the fact that the immune system of human body automatically destroys the beta-cells responsible for insulin production. As a result, in case of T1DM the patient needs immediate insulin treatment to avoid the metabolic breakdown, which starts with hypoglycemic and ketoacidotic state and ends with the death of the patient [2,4]. This is the fact why T1DM is known as insulin dependent DM.
T2DM is the ’Civilization Disease’ form of DM as it is largely related to the wrong or not appropriate nourishment [5]. T2DM is a self-generating process which appears after long term hyperglycemia slowly increasing insulin insensitivity during the years. No insulin injection is needed at the beginning of this type of DM, but in its final un- or poorly-treated stage it needs continuous external insulin treatment to handle the level of glycemia [6,7]. In case of DDM the worst effects of T1DM and T2DM prevail, namely, the autoimmune effects are coupled with increasing insulin resistance. This type of DM has upward prevalence in the recent years, which caused by the double metabolic stress comes from genetic predisposition and lifestyle [8-10].
GDM occurs during pregnancy and most of the cases the DM state ends with the birth, however, those women who suffer from GDM particularly are inclined to emergence of other type of DM in their later life [11,12]. Genetic and the other types of DM can be caused by several disorders [3]. Unfortunately, the occurrence of DM shows an increasing trend. According to the World Health Organization’s study diabetic population will be doubled from 2000 to 2030 exceeding 366 million people [13]. Newer studies have reported that this number is already exceeded and the total count of the diabetic population was around 382 million in 2013, worldwide. Moreover, the projected number of diagnosed and undiagnosed DM cases is going to reach 592 million in 2035 which is the 4.8-6.1% of the estimated total human population on Earth [1,14].
Although DM is still incurable, the therapy of DM presented considerable progression in the recent years, for instance the appearance of different cell stimulating drugs [7,15], partial immunosuppressant drug applications in order to reduce the autoimmune effect [16], transplantation of Langerhansislets [17] or pancreas [18] and stem cell experiments [17] are just some of the achieved results.
The regular treatment for a diabetic patient on insulin treatment is based on external insulin injections. There are two main directions for the insulin administration:
- Insulin administration with insulin pens, which represents the ordinary, conservative therapy;
- Insulin administration with insulin pumps, which is a highly developed electromechanical device.
Both methods have the same purposes from a diabetic patient point of view: keeping the blood sugar level in a narrow, normoglycemic range (3.9-6 mmol/L or 70-110 mg/dL). An important part of these therapies relies on reaching different quality requirements, like body weight, level of physical activation and levels of physiological markers. The main difference is the way how these requirements are achieved. By the appearance of the insulin pump therapy the automated treatment possibility of diabetic patients has created known as the Artificial Pancreas (AP) concept. AP represents today the most challenging engineering tool in the treatment of DM [19]. In the following section, we summarize the AP concept, realization tools and devices, and the engineering considerations behind.
2. Discussion
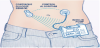
2.1 The concept of Artificial Pancreas In order to realize an AP three necessary subparts is needed [19-21]:
- An insulin pump that stores and injects the rapid acting insulin;
- A Continuous Glucose Monitoring System (CGMS) for continuous blood sugar level measurement;
- Appropriate software components including control algorithms, user interfaces, drivers.
Several insulin pumps are available on the global market [22-24] with different solutions. The basic concept is an ’All-in-One’ type device, where the hardware, mechanical and software subparts are integrated into one device [25]. The other conceptual realization is the divided solution, where important subsystems like the control algorithm or raw data processing are running on an additional device [26,27]. The insulin pump contains an insulin cartridge, a Graphical User Interface (GUI), the battery and the necessary mechanical and hardware elements to inject the insulin and to operate the device. The CGMS system is used in parallel with the insulin pump. The operations of CGMS are based on various principles.
In practice, the most widely used systems are external devices fixed on the abdominal skin surface and connected to the subcutaneous level through a thin catheter. The most frequent measuring principles are enzymatic based (Glucose Oxidase (GOx)). Beside its several benefits CGMS has also some disadvantages mostly from control engineering point of view: sensors measurements are done only every 5 minutes. Implantable CMGS have been also appeared, but these are not available on the market, yet [28].
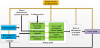
As mentioned above, the third necessary component to realize the AP is the appropriate software elements, including the control algorithms, the ”soul” of this approach. Figure 1 shows the schematic representation of the AP concept.
2.2 Control Algorithms for AP
Due to the fact that insulin pump therapies are used mostly in case of T1DM, the advanced control algorithms developed inside AP researches focus on this DM form. The main expectation from an AP control algorithm is the automatic glucose regulation in order to keep the blood glucose concentration in the normal glycemic range, i.e. 70- 110 mg/dL (3.9-6 mmol/L) and relying if possible on the compliance of the patient. The ultimate goal is to avoid the dangerously low blood glucose levels (massive hypoglycemia) that could directly endanger the patients’ life.
As DM is a widely researched area of biomedical engineering, almost every control method can be found in the literature. The most important directions focus on model predictive control” (MPC), fuzzy rule-based, classical PID control or robust control techniques; however, without having yet a general solution on the problem [19-21]. Simplistically, every control algorithm considers similar principles; namely, the fulfillment of prescribed quality and quantity properties. The first attempts on this area were related to ”Proportional-Integral- Derivative (PID)” control being still the most widely used classical control technique in the industry. Although the basic concept of PID control is not too sophisticated, highly advanced solutions like robust PID [30,31] or switching PID [32] have been applied for the AP concept.
MPC techniques represent probably the mostly used advanced control method in the AP concept, but they suffer from intra- and inter-patient variability and external noises. MPC is a model based solution meaning that the controller tuning is based on the properties of a mathematical model (called nominal model). Nonetheless, MPC algorithms produce the best results in individual therapy with considering closely ideal conditions. Several, highly developed MPC based control solutions appeared in the recent years like Robust MPC (RMPC), Nonlinear MPC (NMPC), Robust, Nonlinear MPC (RNMPC), MPC with moving horizon [33-36].
Soft computing methodologies have been applied also several times in the AP concept, but only in the recent years have been investigated in clinical trials [37-39]. Modern robust control methods like L2- or H∞-based ones were introduced in the AP researches in order to stave off the determinative uncertainties coming from inter- and intra patient variability. Supplemented by Linear Parameter Variability (LPV) methodology (providing the opportunity to handle the original nonlinear system/model as a linear one; hence, to give access using the original nonlinear model for linear control methods enumerated above), modern robust control successfully deals with the quality and quantity requirements [40-43].
Another useful direction in this domain proved to be the combination of LPV methodologies with Linear Matrix Inequalities (LMI)-based one [44, 45]. Its newest direction is connected to Tensor Product (TP) transformations based LMI controller design that is not validated yet in AP, but it can be useful in control of physiological systems [46-48].
Dual hormone controllers consider beside the insulin the glucagon hormone as well; hence, it represents another conceptual control approach in AP researches [49]. Clinical trials also starred in this direction with encouraging results [50].
2.3 Evolution of modeling aspects of diabetes from control perspectives
Controller design applications require a valid mathematical description of the physiological process of glucose-insulin interaction reflected in a mathematical model. The main considerations in the modeling of DM respect to the AP concept are briefly summarized in this section.
Figure-2 emphasizes the key elements to be accounted during modeling and controller development. The modeling of diabetes started with patient models having today highly advanced models available [20].
The directions in this sense can be structured in two parts: inpatient and outpatient therapies. The goals of such models are different, since the circumstances and the environment of the patient are different. The followings give a short review on the evolution of the modeling parts developed from control theoretical perspectives.
Inpatient therapies mostly connect to Intensive Care Unit (ICU), where the strategy is to use as simple patient models as possible. As the patient’s environment is controlled by qualified medical staff and exact protocols, precise glycemic control is possible with prediction of the future condition of the patient as well. In the outpatient concept the patient requires complementary aspects as well, since physiological considerations to be taken into account are more complex then the ICU case; hence, the main expectation from an AP is an efficient control of the glycemic level with uncertain or sometimes unknown/ unrecorded events from the patient.
As a result, in parallel with the patient models additional models were included in the modeling loop in order to give more precised and more realistic physiological (models of digestion, absorption, β-cell, etc.) and physical (models of devices, sensors, etc.) processes.
2.4 The minimal model
As previously mentioned, the first models in the field of AP researches were connected to individual patient models. Their most important example is the Minimal Model (MM) developed by Bergman et al. [51-54]. Several extension of the MM were born in the last decades, however, the key concept remained the same in every case. One of these is the following modification [19]:
Ḡ(t) = -(ρ1 + X(t))G(t) + ρ1GB + ρ(t)
X̄(t) = -ρ2X(t) + ρ3[I(t) - IB]
Ī(t) = γ (G(t)) - h)t - η[I(t) - IB] + u(t)
The model stands from two main parts: the first two equations describe the glucose disappearance, while the third one is for the insulin kinetics. The main idea behind is that the patient gets glucose through p(t) [mg/min] meal absorption rate and the other input is the external insulin injection, u(t) [mU/min]. After a meal the absorbed glucose gets into the blood stream; hence, the blood glucose (BG) level, G(t) [mg=dL] is increasing. If G(t) is different than the pancreatic target glycaemia h [mg=dL], then the β -cells injects insulin to the blood. However, in case of T1DM (γ = 0) where these cells were burned out, the only insulin source is the external injection, u(t) [mU=min]. The connection between glucose and insulin dynamics is the rate of glucose disappearance, X(t) [min-1], which is higher if the level of insulin is increasing. Over the year’s lot of patient models were derived from the MM.
2.5 The ICU models
The ICU models maintain the MM structure, but in a more sophisticated way. The state variables are usually kept in low level ranges while taking into account important kinematic (e.g. decay velocity, diffusion) and physiological (e.g. glucose production of the liver) issues. Important representatives of such kind of models are the Canter bury models [55-60]. However, other models appeared as well [21].
2.6 Outpatient models
The outpatient models developed for the AP concept consider several physiological constraints and effects: the insulin dependent glucose consumption of the tissues whom main energy source is the glucose like muscles; the insulin independent glucose consumption of the nervous system; continuous glucose production of the liver (via the glycogenesis-glycolysis); absorption, diffusion, clearance and decay of the insulin and glucose; the effects of the insulin, etc. Most of these AP models are valid for T1DM due to its general symptom, the total lack of internal insulin production, and operate with high number (6-10) of state variables. One widely used model family was developed by Padova- Virginia group [19,20,61,62]. Based on their researches they created an FDA-approved diabetes simulator as well [63]. Another highly developed solution was created at Cambridge, UK [64,65]. A more complex advanced patient model was published at the MIT Boston USA [66], with more than twenty state variables. Although these models are highly developed, they do not provide solutions to other types of diabetes.
2.7 Model extension to the type 2 diabetes case
Based on the diabetic status of the patient modeling and control considerations occurring in T1DM can be used in the therapy of T2DM patients as well. However, mathematical modeling of T2DM is not a trivial question, since the internal insulin production of the body should be taken into account. Furthermore, it is really hard to identify the diabetic status of the patient. Initially, the insulin production can be really high over years (to compensate the high BG level); nevertheless, with the progression of the disease the insulin production is decreasing and finally, cease with the burnout of the β - cells. Moreover, high inter- and intra-patient variability can be occurred in the amount of the produced insulin because of several environmental causes. Important issue represents the discontinuous secretion of insulin, having rather a pulsatile nature hard to model [67]. That is the reason why only in the recent years T2DM models appeared [45,68-70]. Double diabetic state and the pulsatile nature of internal insulin production was also successfully modeled [70]. The models can be even more precise if the β -cells are also taken into consideration [71].
2.8 Model extension to the type 2 diabetes case
Digestion and absorption models can be essential parts of a patient model as the effects caused by these physiological processes affect directly the variation of the BG level. Digestion models contain more details (i.e. more state variables, considered effects, etc.) as they should describe the whole nutrition procedure (from the meal boluses to the glucose absorption into the blood). Absorption models are simpler because they describe the way of the glucose from the gastrointestinal system into the blood. Usually these kinds of models are focusing only to the carbohydrate (CHO) intake. Highly advanced digestion model was developed by Andreassen et al. [72,73], considering not just the CHO, but also complex meal intakes. The model of De Gaetano et al. [74] and Dalla Man et al. [61,75,76] considered only the CHO intake; however, the model structures are easier to be connected to the AP models having high precision as well. 6) Sensor models: The subcutaneous glucose route and its dynamics represents an important question in the glucose modeling as well, since the commercially available CGMS devices measure interstitial fluid glucose level and use a mathematical model or algorithm to calculate the most likely BG level [77]. Usually the diffusion model is embedded into the patient model [61,64], but it can be placed into the sensor model as well [78,79]. Simple compartmental models are also useful for this aspect of the AP concept [80]. In most of the cases, noise and disturbance models are implemented directly in the above mentioned models. White noise is the basic noise type using with patient and sensor models, while the aging effects of enzymatic based sensors are always treated as disturbances. Finally, regarding validation and testing the importance of the feeding protocol should be emphasized. Due to the fact that the developed solutions should be tested in realistic circumstances, randomized feeding protocols are used. That means that the time frames, amounts and composition of the meal intakes vary during the simulation time.
3. Conclusion
Diabetes Mellitus is an incurable, but treatable disease. For diabetic patients AP could represent probably the most performant solution maintaining their condition in an automatically controlled way. The paper gave an overview of the AP concept, devices, models and the control engineering issues. The clinical trials started in the last years in the AP researches place the concept very close to market applicability and hence to a reality.
Acknowledgments
Levente Kov´acs is supported by the J´anos Bolyai Research Scholarship of the Hungarian Academy of Sciences. Gy¨orgy Eigner thankfully acknowledges the support of the Robotics Special College of the Obuda University. The research was also supported by the Research and Innovation Center of the Obuda University.