1. Introduction
Hyperglycemia associated with maternal obesity or gestational diabetes affects fetal development and influence body composition and health in postnatal life [1]. Regarding skeletal muscle, a key insulin-responsive tissue, fetal stage is crucial for its development because, on general, there is no increase in muscle fiber number after birth [2]. Skeletal muscle account for 40%–50% of body mass, therefore disrupted fetal muscle growth results in low birth weight and impairs whole body glucose and fatty acid metabolism [3,4]. For these reasons the potential mechanisms of influence of extracellular factors associated with obesity and diabetes on muscle growth receive much attention.
Myogenesis involves two excluding processes [5]: myoblast proliferation that occurs through series of events arranged in precise order, i.e. cell cycle, and differentiation concerning the cells that withdraw a cell cycle in G1 phase and are unable to reenter to it [6]. Cyclin-dependent kinase (cdk) 4 and 6 are crucial for regulation of myoblasts proliferation and their inhibition promotes muscle cell differentiation [7,8]. Retinoblastoma protein (pRb) is a cell cycle regulatory protein involved in control of myoblasts proliferation and differentiation [9], since hypophosphorylated pRb binds to and sequesters E2F transcription factors, thus inhibiting the transcription of genes required to G1/S transition [10,11]. Myogenesis requires hierarchical expression of the primary (MyoD and Myf5) and the secondary (myogenin and MRF-4) myogenic transcription factors and results in the morphological changes and muscle-specific gene expression [12].
Our recent observations revealed some modifications of myogenic differentiation under high glucose treatment, manifested by decreased fusion index, myogenin and myosin heavy chain expression as well as an increased level of myostatin [13]. The purpose of the present study was to examine the mechanisms controlling myoblast proliferation, i.e. cellular levels and localization of cyclins stimulating cell cycle progression (cyclin A, B1, D1), the distribution of cyclin D1 in cdk4 complexes, as well as proteins essential for cell cycle arrest and the onset of myogenesis (pRb and MyoD), in mouse C2C12 myoblasts exposed to high glucose.
2. Materials and Methods
2.1 Preparing the high-glucose containing medium
The experimental medium containing high concentration of glucose was prepared directly before using by resolving the appropriate amount of powdered glucose in DMEM (Dulbecco modified Eagle medium), subsequent filtration and supplementation with FBS (Foetal bovine serum) and antibiotic-antimycotic solution (Life Technologies). The final concentration of glucose in the incubation medium amounted to 15 mmol/l and in our early studies six-day differentiation of C2C12 myoblasts under this condition resulted in insulin and IGF-I resistance manifested by the impairment of protein synthesis and protein kinases phosphorylation [14,15]. According to our recent study this concentration of glucose was effective in inhibiting of myogenesis of C2C12 myoblasts, manifested by decreased fusion index and the expression of myogenic regulatory factors: MyoD, myogenin and myosin heavy chain [13].
2.2 Cell culture
Research work was carried out on the murine myogenic C2C12 cell line (satellite cells from thigh muscle, European Collection of Animal Cell Culture, ECACC), that undergoes proliferation and differentiation in response to growth factors present in the extracellular environment, thus, this cell line is a useful model to study the mechanisms controlling myogenesis [16].
Cell cultures were maintained free of contamination at the exponential phase of growth in 10%FBS/DMEM, containing an antibiotic-antimycotic mixture, in controlled humidified air supplemented with 5%CO2, at 37°C. After reaching ~40% confluence, the proliferating myoblasts were subjected to 24-hour exposure to high glucose (15mmol/l) added to 2%FBS/DMEM. The control cells were maintained in 2%FBS/DMEM, which, according to the manufacturer description, contained 5mmol/l glucose, and traces of glucose originated from the serum. To preserve the characteristics of C2C12 cell line, the cells were split up to a maximum of 7 times.
2.3 Assessment of DNA content and cell respiration
A crystal violet (CV) assay was performed to determine the total amount of nuclear DNA (cell proliferation). The cells were cultured in 96-well plates and fixed with 75% and 100% methanol and the monolayer was stained using crystal violet solution (2mg/ml). The excess unbound dye was removed after washing the plates with water. The bound crystal violet was released after adding 1% SDS.
The respiration of the proliferating cells was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT solution (0.5 mg/ml) in PBS was added to each well and the plates were incubated for 4h at 37°C. The reaction product i.e. precipitated formazan, was solubilized in 100% DMSO.
In both assays the absorbance was measured on the Infinite 200 PRO TecanTM multidetection microplate reader (TECAN, Mannedorf, Switzerland) at a wavelength of 570nm.
2.4 Immunoblotting
Whole cell lysates were obtained using RIPA (RadioImmunoPrecipitation Assay) buffer supplemented with protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The protein concentration in the lysates was determined using a BCA kit according to the manufacturer’s instructions. Aliquots of cell extracts corresponding to 100μg of protein were resolved through SDS-PAGE, and subsequently, the proteins were transferred to a PVDF membrane. The membranes were blocked with 5% non-fat dry milk in TBS buffer and incubated with the appropriate primary antibody (obtained from Santa Cruz Biotechnology): rabbit polyclonal anti-cyclin A antibody, (sc-596), rabbit polyclonal anti-cyclin B1 antibody (sc-595), rabbit polyclonal anti-cyclin D1 antibody (sc-718), goat polyclonal anti-cdk4 antibody (sc-260-G), goat polyclonal anti-p21 antibody (sc-397-G), rabbit polyclonal anti-MyoD antibody (sc-760), rabbit polyclonal anti-Rb antibody (sc-50). After three 15-min washes in TBS containing 0.5% Tween 20 (TBST), a 1 hour-incubation with secondary antibody (at 1:5000 dilution) was performed. The secondary antibody was conjugated with the appropriate IR fluorophores, IRDye® 680 or IRDye® 800 CW (IR longer-wavelength near-infrared), for the detection of the specific proteins directly on the PVDF membrane using Odyssey Infrared Imaging System (LI-COR Biosciences). The scan resolution of the instrument was set at 169μm, and the intensity was set at 4, consistent with the standard protocol. The quantification of the integrated optical density (IOD = optical density x area) was performed using the analysis software provided with the Odyssey scanner (LICOR Biosciences). The membranes were also reprobed with antiactin antibody (goat polyclonal, sc-1616, Santa Cruz Biotechnology), to ensure that all lanes contain equal amounts of total protein.
2.5 Immunoprecipitation
To assess potential protein interaction and protein distribution in complexes, the stage of immunoprecipitation previous to immunoblotting was used. Whole cell lysates containing 300μg of total protein were subjected to 12-hour incubation with anti-cdk4 antibody previously adsorbed on Protein A/G Plus agarose (sc-2003, Santa Cruz Biotechnology). The antigen-antibody complexes adsorbed on the agarose beds were recovered by centrifugation, washed 3 times with PBS, and then subjected to SDS-PAGE, the electrotransfer and blotting with anti-cyclin D1 antibody, as was presented in our recent study [17]. The control probing with the antibody used for immunoprecipitation was also performed, to ensure that equal amounts of protein were precipitated and recovered from whole cell lysates.
2.6 Immunofluorescence staining and confocal microscopy
The cell cultures were carried out directly on glass Lab-tec coverslips (Nunc Inc., USA). The cells were fixed with 3.7% paraformaldehyde for 20min at room temperature and permeabilized with 0.05% Triton X-100 in PBS. The cells were incubated overnight in darkness at 4°C with the primary antibody (listed in the immunoblotting subsection, Santa 7 Cruz Biotechnology) diluted 1:100 in PBS. The slides were rinsed three times with PBS and incubated for 1 h with Alexa Fluor 488 secondary antibody (Eugene, USA) diluted 1:500 in PBS. For nuclear visualization, the cells were stained with 7-aminoactinomycin D (7-AAD, 5μg/ml) in PBS for 15 min at room temperature. After rinsing, the coverslips were mounted on glass slides using Fluorview mounting medium (Sigma-Aldrich) and the cells were visualized by confocal laser scanning microscope FV-500 system (Olympus Optical Co, Hamburg, Germany). The combination of excitation/ emission were: Argon 488nm laser with 505-525nm filter for Alexa Fluor 488 and HeNe 543nm laser with 610nm filter for 7AAD nucleus staining. Stack of cross-sections were gathered separately for each fluorescence channel. Ten independent fields from each repetition of control and experimentally-treated cell cultures were photographed and the integrated optical density (IOD) values were measured using MicroImage analysis system (Olympus Optical Poland). To estimate the nuclear localization of examined proteins, circles were drawn around every nuclei with red channel and then pixels inside the circles were counted from green channel. The total fluorescence of fields with similar cell density reflects the green fluorescence measured both in green and red channel.
2.7 Statistical analysis
The results of the CV and MTT tests are representative of four separate experiments performed in triplicate (n = 12). The data obtained from the immunoblotting analysis represent three separate experiments performed in triplicate (n = 9). The experiments visualizing the cellular localization of examined proteins were performed three times with 3 wells/treatment. Ten randomly selected non-overlapping fields with similar cell density were photographed. The individual data (n = 10) used for statistical analysis represent the total fluorescence and calculated nucleus/cytoplasm fluorescence ratio. All results were presented as means ± SE. For each assay, Student t-test was used to compare the two means (control vs experimental treatment) and the criterion for statistical significance was P<0.05. The analyses were performed using GraphPad Prism 5 (GraphPad Software, USA).
3. Results
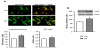
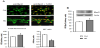
The C2C12 myoblasts exposed to high glucose for 24 hours proliferate faster than control, as manifested by significant increase in the crystal violet test value (by 19% in comparison to control, p=0.027, Table 1). High glucose also caused an increase in cell respiration (by 24% over control value, p=0.004), probably resulting from increased myoblast number in experimentally-treated cell cultures. Cyclin A was present both in the cytoplasm and in the nuclei of the proliferating myoblasts, this latter was manifested by yellow fluorescence, resulting from simultaneous excitation of green (CycArelated) and red (nuclear 7-AAD) fluorochromes (Figure 1A). High glucose supplementation augmented the total CycA fluorescence (P<0.0001 vs control) and caused a slight increase in nuclear CycA localization (P=0.045 vs control). The increase in the cellular level of CycA in HG-treated myoblasts was confirmed by immunoblotting (P=0.0002 vs control, Figure 1B).
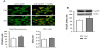
High ambient glucose augmented the level of cyclin B1 in myoblasts, as assessed by immunofluorescence (P=0.007) and by immunoblotting (P=0.0005 vs control, Figure 2). Exposure of myoblasts to high glucose did not markedly altered the cellular localization of cyclin B1 (P=0.55).
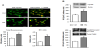
In control cultures cyclin D1 was localized both in cytoplasm and in myoblast nuclei (Figure 3A). High glucose markedly augmented the level of cyclin D1 in myoblasts (P<0.0001 vs control), and this increase concerned predominantly the nucleus (strong yellow fluorescence, and higher N/C ratio, P<0.0001 vs control). An increased level of cyclin D1 in myoblasts treated with high glucose was confirmed by immunoblotting, moreover cdk4- bound CycD1 was also augmented by HG (Figure 3B).
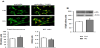
The cellular content of pRb was slightly but significantly increased under HG treatment, as was proved by immunofluorescence (P=0.005 vs control) and immunoblotting (P=0.002 vs control, Figure 4). High glucose altered the cellular pRb localization in myoblasts, i.e. it decreased an abundance of this protein in myoblast nuclei (P=0.03 vs control).
Supplementation of growing medium with high glucose led to slight increase of cellular content of MyoD (P=0.005 and P=0.0004 vs control, for immunofluorescence and immunoblotting, respectively, Figure 5) however a nuclear localization of MyoD decreased markedly under the experimental condition (P<0.0001).
4. Discussion
The purpose of the present study was to examine the regulation of myogenic cells proliferation by high glucose, one of humoral factors associated with insulin resistance and diabetes. Currently, relatively little is known about the mechanisms controlling muscle cell growth under high glucose treatment. High glucose was known to induce proliferation of vascular smooth muscle cells, acting through NFkB- dependent transcription of growth factors and augmenting of E2F-1 transcription factor essential for cell cycle progression [18]. Nedachi et al. [19] have found that the ability of insulin to stimulate myogenesis is affected by extracellular glucose, i.e. insulin exerted its myogenic effect only in the presence of high ambient glucose.
In our study high glucose present in extracellular environment increased growth rate of proliferating myoblasts, assessed by crystal violet test (Table 1) and this effect was accompanied by augmented cellular level of G1-cyclins important for cell cycle progression. The increase in cyclin A, its nuclear localization manifested by yellow fluorescence and the increase in nucleus/cytoplasm ratio of CycArelated fluorescence in glucose-treated myoblasts (Figure 1), allow predicting the increase in the cyclin activity. Similarly, in myoblasts treated with high glucose the increased level of cyclin D1, particularly in the nucleus, was observed (Figure 3). Previous work has established cyclin D1 as a predominant cyclin that control the rate of progression through the G1 phase of the cell cycle [20]. Moreover, majority of the cyclin D1 activity was associated with cdk4 in cultured mouse fibroblasts and muscle cells [21]. In the present study, cyclin D1 was bound with cdk4, and exposition of myoblasts to high glucose augmented this fraction of cyclin D1.
We found only slight but significant increase in cellular content of pRb and shift of cellular localization of this protein toward cytoplasm in high glucose-treated cells (Figure 4). Such an observation suggests a role of pRb in the mitogenic effect of high glucose in myoblasts. We hypothesized that increased CycD1-Cdk-4 activity augmented phosphorylation of pRb, which was manifested by appearance of slowly migrating isoform(s) of this protein revealed by immunoblotting. In this phosphorylation state pRb did not sequester transcription factors required for cell cycle progression [11]. Interestingly, MyoD is likely to be involved in the growth promoting effect of high glucose, since this factor, although augmented, was localized mainly in the cytoplasm of experimentally-treated myoblasts (Figure 5). According to Ferri et al. [22] the activation of MyoD is associated with its nuclear localization. In myoblasts stimulated to proliferation cyclin-dependent kinases can phosphorylate myogenic regulatory factors, among the others MyoD, and promote their inactivation, thereby suppressing the differentiation program (summarized in [23]).
In the previous study we supported the hypothesis that expression of key myogenic regulatory factors, IGF binding proteins and important extracellular matrix components could be targeted by high glucose in differentiating myocytes [13]. Present results indicate the subsequent mechanism of the modulatory effect of high extracellular glucose on skeletal muscle growth. In conclusions, stimulation of C2C12 myoblasts proliferation by high glucose is associated with: i) an increase of cyclin A and cyclin D1 in myoblast nuclei, and formation of cyclin D1-cdk4 complexes, ii) a decrease of pRb and MyoD nuclear localization. These alterations can lead to increase in number of myoblasts contributing to formation of muscle fibers.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
KG and KGK: research design, experimental design; KG, MB and MM: cell culturing, CV and MTT tests, immunoblotting and immunoprecipitation analyses; MG: immunofluorescence and confocal microscopy; KGK: manuscript and figures preparation. All authors read and approved the final manuscript.