1. Introduction
Obesity is a major pandemic in the United States and across the globe. Currently 35% of adult men and > 40% of adult women in the United States are obese and two-thirds of the adult population is considered either overweight or obese [1-3]. Obesity is associated with high rates of cardiovascular disease (CVD) and mortality, and an increased prevalence of CVD risk factors such as hypertension, diabetes mellitus (DM), dyslipidemia and obstructive sleep apnea[4-8].
While diet and exercise strategies have been shown to reduce weight and CVD risk factors, these lifestyle modifications are difficult to sustain and many of these patients will regain lost weight at 1-year follow-up [9]. Furthermore, lifestyle modifications have failed to curb the rising epidemic of obesity. Bariatric surgery has also shown a benefit in sustained weight reduction, improved in CVD risk profiles and co-morbid conditions such as DM, hypertension and dyslipidemia, and reduced mortality. However these procedures are invasive and not universally available, and predispose patients to risks such as perioperative morbidity and mortality, gastric obstruction or ulceration, infection, bacterial overgrowth and malabsorption [10].
While pharmacological weight loss is more practical and widely available, long-term effects on weight reduction and incidents of CVD remains largely unclear [11]. Over the past decade several weight loss therapies such as orlistat, lorcaserin, phentermine/topiramate and naltrexone/bupropione have emerged as potential safe and effective agents for pharmacotherapy in the treatment of obesity [12-17].
We conducted a systematic meta-analysis to assess the effects of pharmacological therapy for the treatment of obesity, on reduction on CVD, CVD outcomes and all-cause mortality.
2. Methods
Anti-obesity drugs that were studied included orlistat, lorcaserin, Phenteramine/topiramate, Naltrexone/Bupropion. Using the key words , “orlistat”, “lorcaserin”, “phentermanie/topiramate”, “naltrexone/bupropion”, “cardiovascular”, “cardiovascular outcomes”, “cardiovascular risk factors”, we surveyed the PubMed, Trial search of Cochrane Library and Web of Science databases, Figure 1.
Included studies in our meta-analysis fulfilled the following requirements: all studies had to be published in peer-reviewed journals, could be either prospective or retrospective studies and contain quantitative data on the effect of anti-obesity drug on cardiovascular risk factors including Hemoglobin A1c (A1C), fasting blood glucose, BMI, weight, systolic and diastolic blood pressures, total cholesterol, LDL:HDL ratio, LDL cholesterol, HDL cholesterol, triglycerides; CVD outcomes including myocardial infarction, stroke and/or mortality.
Accepted controls identified in the studies were placebo with hypocaloric diet, placebo with maintenance diet, hypocaloric diet alone, placebo alone, hypocaloric diet with moderate exercise regimen, and placebo with an Internet based weight management program including healthy diet and exercise.
Exclusion criteria included studies that were not randomized controlled trials and duplicate studies that drive data from the same trials.
791 studies were screened initially, 744 were excluded because they were not randomized controlled trials. Out of 47 studies that were randomized controlled trials, 7 studies fulfilled the inclusion criteria.
Outcomes compared between the intervention (INT) and control (CTRL) groups included all cause mortality (ACM), cardiovascular mortality (CVM), absolute A1C reduction, percent weight reduction (WT%) and absolute systolic blood pressure reduction (SBP). ACM and CVM were compared and reported using events and odds ratio respectively. A1C, WT% and SBP were compared and reported using mean difference and hedges’s g (Hg). The data were analyzed using the Comprehensive Meta-Analysis package V3 (Biostat, USA). Mantel- Haenszel method [18] was used for calculating the weighted pooled odds ratio under the fixed effects model. Heterogeneity statistic was incorporated to calculate the summary odds ratio under the random effects model [19].
3. Results
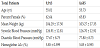
There were a total of 7 studies included in the final analysis, this included a total of 18598 subjects, of which 8685 were in the INT group and 9913 in the CTRL group. Baseline demographic data for the study cohorts is listed in Table 1.
There was a significant absolute reduction in A1C for the INT group (Hg: -0.238, 95%CI: -0.291 - -0.186, Z: -8.937, P< 0.001). The WT% reduction was significantly higher for the INT group compared to the CTRL group (Hg: -0.431, 95%CI: -0.477 - -0.385, Z: -18.472, P< 0.001) and the SBP reduction was higher for the INT group compared to the CTRL group. (Hg: -0.052, 95%CI: -0.101- -0.003, Z: -2.086, P: 0.037). The heterogeneity observed for our meta analysis is Q: 1.884, df: 6, P: 0.930. Figure 2 represents the meta analysis and results for the final outcomes.
There was no significant difference in ACM between groups, 45 events in the INT and 55 in the CTRL (OR: 0.843, 95%CI: 0.571- 1.244, Z: -0.860, P: 0.390), however there were significantly fewer CVM events the INT group , 17 events in the INT and 36 events in the CTRL suggesting CVM benefit (OR:0.496, 95%CI: 0.282-0.873, Z: -2.433, P: 0.015). There was a significant absolute reduction in A1C for the INT group (Hg: -0.238, 95%CI: -0.291 - -0.186, Z: -8.937, P< 0.001). Figure 2 represents the meta analysis and results for the final outcomes.
4. Discussion
Our study demonstrated favorable and significant effect of pharmacological weight reduction strategies on weight loss, blood pressure reduction, glycemic control (A1C reduction), and CVM. While these findings are consistent with the effects of surgical weight reduction,[19,20] our study adds to the limited literature on the CVD effects of pharmacological therapy for obesity.
Weight loss has been noted to modify risk factors via improving insulin sensitivity, reducing inflammation, decreasing blood pressure and modifying the lipid profile [21-24]. The mechanism by which weight loss medications impact CVD risk reduction could be either a direct effect of these agents or merely an effect on weight reduction. Without pharmacological interventions, diet and exercise regimens when successful in reducing weight have been shown to reduce CVD risk [25,26]. Additionally, whether via lifestyle modification or bariatric surgery, weight loss without pharmacological therapy has been shown to decrease CVD risk [27,28]. This would support the notion that CVD risk reduction by pharmacological agents is largely due to the effect of weight loss itself. Furthermore, the mechanism of action of these medications are not directly anti-inflammatory, and do not directly modify insulin sensitivity, blood pressure or the lipid profile [29-32].
Thus, it is most likely the benefit on CVD from these therapies is via weight reduction and not direct medication effect [29-32].
One limitation to our analysis was the small number of long-term studies assessing the CVD risk factors and mortality with each of the medications we evaluated. Thus, our meta-analysis could not compare the CVD outcomes between individual pharmacological agents. In addition, many studies did not contain enough information to assess non-traditional risk factors such as inflammatory markers or assessment of dyslipidemia.. We also found there was no decrease in all-cause mortality in our study, potentially due to medication impact on the gastrointestinal, hormonal or neurological systems, which were beyond the scope of our meta-analysis.
In summary, through our study demonstrated that use of pharmacological weight loss therapy is beneficial in weight reduction, improvement of A1C and blood pressure and decreases rates of CVM when compared to placebo and diet/exercise regimens. This suggests that pharmacotherapy maybe a treatment option for CVD risk reduction in obese patients. Further research should be performed to clarify the implications these therapies have on overall mortality and evaluate the mechanisms by which these medications reduce CVD risk factors and mortality.
Competing Interests
The authors declare that they have no competing interests.