1. Introduction
CPC is an unusual malformation consisting of a shortened colon combined with a pouch like dilatation associated with anorectal agenesis usually associated with a genitourinary fistula.
This condition has been classified by Narasimharao et al. [1] into 4 groups according to anatomical subtypes based on the length of colonic pouch in relation to normal colon. Subsequently extended ways of classification were published for example defining pouch colon in complete congenital pouch colon (CCPC) and incomplete congenital pouch colon (ICPC) [2]. The “Saxena-Mathur classification” categorizes CPC into 5 types regarding the anatomical morphology [3].
Due to variability of anatomical features and different clinical manifestations, there is a wide range of surgical treatment options of the CPC. Despite the possibility of using staged procedures with protective placement of stoma or using one stage procedure, two different main surgical strategies are discussed in literature. The first one is pouch resection and pull through procedure. The second strategy is tubularisation of the colonic pouch and pull through of the coloplasty [4-11].
We describe our surgical strategy in three cases and outcome of these patients.
2. Patients and Methods
All patients had a rare variant of CPC malformation and closely resembled each other with ileum opening directly into the colonic pouch (CCPC), without a genitourinary fistula.
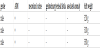
They have been transferred to our institute after birth with the initial diagnosis of anorectal malformation with imperforate anus in a time period of ten years (2001-2011). None of them had prenatal screening tests or ultrasound examinations during pregnancy. Clinical examination revealed distended abdomen and perineal inspection showed no cutaneous fistula. Meconium was not detected in the urine (Table 1. Patient`s characteristic). Based on further evaluations with ultrasound investigation and abdominal plain x-ray CPC anomaly was suspected.
Transverse laparotomy was performed and exploration confirmed CPC in all patients. Because ileum opened directly into these colonic pouches we decided to perform a diverting ileostomy to save the colonic pouch for the second stage procedure. Approximately 15 cm proximal of the colonic pouch, a diverting stoma was created. With intra-operative contrast medium imaging of distal intestinal segment, we could verify that colonic pouches indeed had no connection with bladder or urinary tract (Figure 1).
There was no further associated gastrointestinal anomaly.
The distal tube of the ileostomy was irrigated twice a week with normal saline solution. After at least 6 months all three patients thrived well and no complication occurred during this period. As second stage operation stapler tubularized coloplasty was performed (Figure 2).
After closure of the laparotomy incision, reconstruction of intestinal continuity was carried out by abdominal posterior sagittal “anocoloplasty” comparable to PSARP (posterior sagittal ano-rectoplasty) (Figure 3).
Postoperative wound care of the "neoanus" was performed with dressing material. Starting from the second postoperative week we performed anal dilatation protocol according to Pena guidelines [12] continuing for at least six months.
Subsequently, after complication-free healing process the diverting ileostomy was closed with end-to- end single layer anastomosis.
3. Results
None of the children had perioperative surgical complications. Only one patient suffered from excoriations circumferential in the area of the "neoanus", which could be healed by local treatment with zinc ointment.
Initially the frequency of bowel movement was 8 to 7 per day, but it decreased to just four to two times daily. After three months all patients had semi formed stools. Two patients had postoperative clinical follow-up for three years and the third patient for only two years. None of the patients developed long term complications, neither pouch re-dilatation nor anoplasty stricture.
During the entire follow-up period these children thrived well and had permanently semi formed stool two to four times per day.
4. Discussion
Congenital pouch colon is a rare variant of anorectal malformation described in the literature with 9% [7] and 6.3% -15.1% [9]. Our three cases represent a very rare form of CPC type I [1] without genitourethral fistula. In the literature Mathur et al. described one case out of 80 [4], Chadha et al. 3 cases out of 41 [7] and Sharma et al. 3 out of 68 cases [11].
Large series of the CPC anomalies were reported mostly from India and neighboring nations in Asia pointing out the high complication potential of surgical management and functional outcome.
Many of these reports addressed possible reasons for severe complications like re-dilatation of the tubularized colonic pouch after pull-through surgery [4,6,8,10,11]. Gangopadhyaya et al. reported in their large series on the histopathologic examinations of the resected pouches and pointed out that these structures have abnormally developed tissue and need to be resected for better functional outcome [9]. Other authors focused on the surgical technique performing tubular colorraphy [5,8,10]. With our technique of creating coloplasty tube we reiterate these recommendations concerning size and length of colonic tube and fortunately we did not see this re-dilatation phenomenon during our observation period.
Stricture of the anoplasty after PSARP is a well-known complication and this kind of stricture might influence the formation of a re-dilatation of the tubularized colon. Therefore in our opinion long-term dilatation of the "neoanus" is a very important fact. We provided close outpatient follow-up of our patients keeping strictly to the dilatation protocol according to Pena [12] for at least 6 months. Overall we assume some selective advantage for PSARP surgical procedure due to a lack of genitourinary fistula in our selected small patient collective.
Main functional postoperative problems of the children with CPC are high frequency bowel movements with aqueous stools, high stool frequency and perineal excoriations. All these gastrointestinal complications may cause a failure to thrive and a loss of quality of life. Poor continence with high stool frequency was described after pullthrough of ileum in patients with CPC type I/II [6]. Our intention to retain a tubularized pouch colon was to reach continence to a certain extend in contrast to ileum pull through. Further we intended to avoid constructing a technical complex J-shaped or S-shaped ileum reservoir and wanted to prevent a permanent abdominal enterostomy.
However despite the fact of already published large series in literature the decision for choosing the adequate surgery method and subsequently achieving a good quality of life for these patients is not easy.
In conclusion concerning the very rare variant of CCPC without genitourinary fistula, we can speculate that coloplasty with posterior sagittal “ano-coloplasty” followed by anal dilatations works well, and that these patients may have a fairly good outcome. Nevertheless it should be reevaluated in the long term.
Competing Interests
The authors declare that they have no competing interests.