1. Introduction
Coagulopathy is a frequent complication after prolonged use of cardiopulmonary bypass (CPB) and usually requires transfusion of various blood components in cardiac surgery [1]. However, considering the risk of transfusion-related complications and postoperative morbidity and mortality [2], accurate and fast determination of the whole-blood coagulation profile and the etiology of any associated coagulopathy are necessary to avoid unnecessary transfusions and or to minimize their size [3].
Standard laboratory-based tests (SLTs) cannot carry out timely and effective management of concurrent coagulopathies due to their inability to reflect the whole-blood coagulation profile and long turnaround time. Therefore, implementation of a reliable and predictive intraoperative point-of-care (POC) coagulation test that can diagnose various types of coagulopathy in a much faster and customized manner would facilitate intraoperative coagulation management. This in turn could help avoiding unnecessary transfusions, prompt transfusion of essential component(s) for managing concurrent coagulopathies, and prevent consumptive coagulopathy due to delayed transfusion in massive bleeding.
In the present case, implementation of a routine intraoperative rotational thromboelastometry (ROTEM) assay immediately after weaning from CPB enabled earlier diagnosis of unexpected hyperfibrinolysis and hypofibrinogenemia and facilitated timely and appropriate management consisting of anti-fibrinolytic therapy, and cryoprecipitate transfusion during cardiac surgery.
2. Case Report
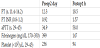
A 54-year-old male underwent an elective aortic valve repair and graft interposition procedure for a severe aortic regurgitation and dilated ascending aorta tubular portion (diameter 5.4 cm). He had been taking diuretics, beta-blocker and clopidogrel 75mg daily before surgery. In preoperative hematologic evaluation, he had a leukopenia and normocytic anemia with white blood cell of 2810 count•-μL-1 and hemoglobin (Hb) of 12.7 g•dL-1, but his activated partial thromboplastin time (aPTT), prothrombin time (PT), and PT international normalization ratio (INR) were normal (Table 1).
CPB with moderate hypothermia (28°C) was employed for aortic valve repair and graft interposition in the dilated ascending aorta. Intravenous heparin was administered and supplemented to achieve an activated clotting time (ACT) of 450 s and to maintain ACT > 500 s during CPB.
He was weaned from CBP 170 minute after achieving stable cardiovascular performance, as indicated by hemodynamic parameters including arterial pressure (BP), heart rate (HR), central venous pressure, pulmonary artery pressure, the thermodilution cardiac index and transesophagesl echocardiography examination. Five minutes after the completion of the protamine infusion to neutralize the effects of heparin, ACT and ROTEM (ROTEM, Tem International GmbH, Munich, Germany) assays including INTEM, EXTEM, FIBTEM, and APTEM were started as part of the routine coagulation management protocol at our institution. ACT was 139 s (130 s before CPB). Until 10 min after starting the ROTEM assays, the tracings showed the following patterns: a reduction in the α-angles in INTEM, EXTEM, and APTEM; substantially increased clot formation time (CFT) in INTEM (248 s; reference value 30–110 s), EXTEM and APTEM (246 s and 637 s, respectively; reference values 34–159 s in both); and significant reductions in the amplitudes at 10 min (A10) and 20 min (A20) of INTEM (31 and 34 mm, respectively; reference values 44–46 mm and 50–71 mm, respectively), EXTEM (33 and 36, respectively; reference values 43–65 and 50–72 mm, respectively), APTEM (19 and 28 mm, respectively; reference values 43–65 and 50–72 mm, respectively), and FIBTEM (3 and 3 mm which indicate hypofibrinogenemia, respectively; reference value >7 mm in both). Simultaneously, there were massive ongoing bleeding from aortic root and abruptly decline of systemic BP. At that time, Hb was 5.9 g•dL-1 and the ROTEM assays showed hyperfibrinolysis status: the INTEM and EXTEM tracings showed abrupt reductions in clot lysis index at 30 min (CLI30) (60% and 65%; reference value 94–100% in both) and their further reductions at 45 min (CLI45) (0% and 12%; reference values 94–100% in both); the APTEM tracing showed constant amplitude without any decrease in CLI30 or CLI45 (Figure 1). At the same time, the amplitudes of ongoing INTEM, EXTEM, and FIBTEM tracings became undetectable due to complete resolution of clots by concurrent hyperfibrinolysis.
Based on these ROTEM assay, 2.0 g tranexamic acid (TA) was administered intravenously, 30 minutes the start of the ROTEM assay. In the same time, cryoprecipitate was prepared in addition to transfusions of platelet concentrate, fresh frozen plasma (FFP), and packed red blood cell (RBC). During the administration of TA and the thawing and preparation of the blood products, additional bleeding in the patient’s nostrils was noted. In addition, the amount of bleeding, which determined indirectly based on the speed of intravenous volume resuscitation (reaching around 100 mL•min-1) by a rapid infusion device, gradually increased.
An additional ROTEM assay was initiated 10 min after the completion of TA administration (Figure 2); it showed restoration of clot lysis function (indicated by normal CLI 30 and CLI 45), although clotting factor deficiency, hypofibrinogemia and thrombocytopenia continued (indicated by the reduced α-angle, prolonged CFT and reduced A10 and A20 in EXTEM), likely due to ongoing massive bleeding.
After additional transfusions with FFP (2 units), platelet concentrate (8 units; about 300 mL), RBC (4 units) and cryoprecipitate (8 units), surgical bleeding was reduced dramatically and the speed of intravascular volume resuscitation for maintaining stable hemodynamics became 10–15 mL•min-1.
Additional RBC (3 units) was transfused after the follow up laboratory result of Hb 7.5 g•dL-1. In the ICU, additional RBC (2 units) and FFP (6 units) were transfused and the post-operative bleeding reduced. 1 post-operative day follow up ROTEM assay showed all parameters within normal range.
3. Discussion
Blind coagulation management without POC guidance can underestimate the real demand for coagulation factors in severe bleeding [4]. Severely bleeding patients need goal-directed coagulation management using a quick and reliable coagulation monitor as well as a targeted therapeutic approach specific to the test results [4]. Commonly used SLTs including PT, APTT, and fibrinogen assays are time-consuming compared to ROTEM assay (53 min vs. 23 min) [5]. Furthermore, PT and aPTT tests do not reflect the underlying etiology of a complex coagulopathy [6,7]. In contrast, goal-directed coagulation management using ROTEM facilitates an accurate and timely assessment of clot initiation and the quality of established clots [8], by graphically indicating the initiation of coagulation, clot formation, and strength beyond the initial fibrin polymerization (including ACT, α-angle, and CFT), and clot stability (including lysis index and maximum lysis) to diagnosis fibrinolysis status.
Previous studies have demonstrated the efficacy of POC coagulation monitoring for guiding hemostatic management after CPB and reducing transfusion and hospital mortality in cardiac surgery [9-11]. The most recent European guidelines [11] also suggest that routine use of POC analyses, such as ROTEM, would help to reduce transfusion requirements, improve outcomes, and lower costs: parallel INTEM, EXTEM, FIBTEM, and APTEM assays allow prompt and appropriate management of coagulopathy in a more customized manner using each component in the coagulation cascade (e.g., fibrinogen, coagulation factor, platelets, and anti-fibrinolytic therapy). Massive bleeding due to hyperfibrinolysis usually leads to further deterioration of coagulation. Furthermore, massive volume replacement using banked and intraoperatively salvaged red blood cells produces further dilution of the plasma components for maintaining coagulation (Figure 1).
Above all, the present case showed that the routine use of multiple ROTEM assays immediately after CPB enabled not only earlier and precise diagnosis of unexpected hyperfibrinolysis with hypofibrinogenemia superimposed on the usual CPB-induced bleeding diathesis within 30 minutes after the initiation of ROTEM assays [12], but also timely anti-fibrinolytic therapy using TA to reduce bleeding and transfusion requirements. Without this prompt diagnosis, much greater consumptive bleeding, requiring a much greater amount of transfusion would have persisted in the present case. Considering that anti-fibrinolytic therapy should be selectively recommended due to its possible adverse effect producinghypercoagulable status [11], ROTEM may be useful to determine the timing of this therapy.
In the present case, hypofibrinogenemia and lack of other factors for the clot formation cascade were promptly diagnosed by MCF < 7 mm in FIBTEM and managed by timely transfusion of cryoprecipitate. Fibrinogen is the first factor that is typically depleted during massive bleeding and hemodilution [13] and suggested to maintain 1.5–1.8 g/L [11,14]. Meanwhile, the conventional indirect Clauss method is not reliable to determine the fibrinogen level in the use of heparin as in the present case (Figure-2).
A large volume of FFP (15–20 mL•kg-1) for restoring fibrinogen to an appropriate level is also problematic [11,15]. Therefore, ROTEMguided administering fibrinogen concentrate (FC) or cryoprecipitate can be more sophisticated management of hypofibrinogenemia in patients undergoing cardiac surgery. However, FC is not available in most countries, as in the present case.
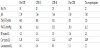
Body temperature and acid–base balance were well maintained, and hypocalcemia, probably due to the massive transfusion, was supplemented by intravenous calcium chloride during the post-CPB period (Table 2). The effect of preoperative clopidogrel on platelet function, which could not evaluated by ROTEM alone, was not evaluated by other POC aggregometry in the present case. A slight reduction in amplitude in EXTEM during the post-CPB period was treated by empiric platelet transfusion without confirmation of platelet function or count.
4. Conclusion
In conclusion, the present case shows that the implementation of routine parallel ROTEM assays immediately after CPB enables earlier determination of unexpected post-CPB bleeding diathesis and facilitates timely and appropriate coagulation management in cardiac and major vascular surgery.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
This case performed in the Konkuk University Medical Center, Konkuk University School of Medicine.