1. Introduction
A dual-task paradigm has been widely used to understand the attentional demands of balance and locomotor tasks. The view that maintaining stability during upright postural tasks may involve attentional resources stems from several empirical findings showing that the performance on the postural and/or cognitive task declines when the two are performed simultaneously [1-4]. This is referred to as cognitive-motor interference (CMI) [1]. These findings can be interpreted in the light of two main frame works - “capacity sharing” and “bottleneck” theories which assume that the central resources/ capacity is limited resulting in a processing delays when two tasks requiring similar neural pathways are performed concurrently.
While evidence shows that balance and locomotor tasks are attentionally demanding, the innate demands of the motor task itself could affect the performance in a dual-tasking situation. For example, attentional demands for reaching from a standing position involving voluntary action however, limited movement across different body segments on a stable base of support (BOS), could differ from the demands of walking involving movement across several body segments through a changing BOS. Further, attentional demands may vary depending on threat perceived and the level of experience/skill to perform a specific task. Thus, maintaining balance while walking on an even surface could incur lower attention demand than that while catching balancing during sudden disturbance causing slip or a trip. Limited studies have examined the differential attentional needs of balance activities. One study by Lajoie et al. [2] demonstrated longer reaction times to an auditory stimulus during static standing as compared with static sitting suggesting postural tasks with greater balance demands also needs greater attentional resources.
As such, cognitive load during balance tasks also affects younger and older adults differently. Sparrow et al. demonstrated that older adults have greater reaction times to a visual task while walking or greater decline in gait speed while performing a memory recall task as compared with younger adults [3,4]. A direct injury to the central nervous system such as a stroke, Parkinson’s disease or multiple sclerosis resulting in both motor and cognitive impairments also seems to affect dual-tasking ability as these individuals need to maintain balance in the face of reduced neural resources and sensorimotor impairments [5,6]. Cognitive and motor deficits occur with aging and neurological conditions. As presence of cognitive deficits affect motor abilities, it is essential to understand attentional demands of different balance tasks involving intentional and reactive balance in individuals aging with and without neurological conditions as well.
Therefore, the purpose of this study is to examine the influence of a single higher cognitive (working memory) task while concurrently performing three different dynamic postural tasks – limits of stability (intentional balance), compensatory stepping during large magnitude backward perturbations (reactive balance) and walking across healthy younger adults, older adults and older chronic stroke survivors. Motor and cognitive cost of performing the three postural tasks under dualtask conditions was measured. Based on the attentional demands incurred by the postural tasks, the motor costs, cognitive or both the costs will differ across the three balance tasks within all the groups. Further, motor and cognitive costs of performing the balance tasks would be greater among stroke survivors as compared with healthy adults.
2. Materials and Methods
2.1 Participants
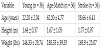
Healthy young adults (18-30 years, N = 36), community dwelling chronic stroke survivors (50-70 years, N =36) and age-matched healthy adults (50-70 years, N = 36) participated in the study. The information about type of stroke was obtained from subjects’ physicians. Stroke survivors were included if they satisfied the following criteria 1) ability to walk 10m with a speed of ≥ 0.58 m/s without any assistive device i.e., least limited and unlimited community ambulators and 2) intact cognitive function determined by score of ≥ 26/30 on Montreal Cognitive Assessment scale. This test focuses on different aspects of cognitive functions such as orientation, attention, recall, working memory, and language and the stroke survivors were excluded in presence of any other acute or chronic medical conditions. Table 1 shows subject demographics for all the participants. Performance on measures of balance – Berg Balance Scale, motor impairments – Chedoke McMaster Stroke Assessment and physical activity levels using Rapid Assessment of Physical Activity was assessed for stroke survivors (Table 2). The healthy adults were excluded if they presented with any acute or chronic musculoskeletal, neurological and cardiopulmonary conditions. In addition to stroke survivors, younger and age-matched healthy controls were included to examine the effect of both aging and chronic stroke on CMI. The CMI pattern was assessed for all subjects performing one of the three different balance tasks.
2.2 Intentional balance assessment
Intentional balance was assessed using the limits of stability test (LOS) by NeuroCom SMART Equitest for computed dynamic posturography. The subjects donneda harness and stood on the platform placing their feet on the force plates. The subjects’ center of pressure vector was projected on the screen in front of them in the form of a figure, known as “avatar”. The subjects attained the initial position to maintain the avatar in the center of the screen. Upon receiving an auditory cue, subjects moved towards the target in the forward direction as fast as possible. Subjects are asked to reach as close to the target as possible and hold the avatar in that position until the second auditory cue was heard while refraining from stepping or holding onto the surrounding box. After receiving a single familiarization trial, all the subjects performed this task in single-task and dual-task conditions.
Outcome: The maximum excursion of the center of pressure (MXE) in the forward direction was recorded [7]. This indicates the maximum distance up to which the individual can shift his/her center of mass outside the base of support without initiating a step, reflecting the individual’s limits of stability in the forward direction.
2.3 Reactive Balance Assessment
The subjects were exposed to trip-like perturbations from standing position on a motorized treadmill, ActiveStep (Simbex, Lebenon, NH). Initially subjects assumed a comfortable stance on the treadmill with their feet shoulder width apart. A harness donned prevented the participants’ knees from touching the treadmill in case of a fall. Prior to the testing session, the participants were presented with a familiarization trial wherein they were instructed to execute a natural response to recover their balance upon a sudden backward trip-like perturbation. This trial was presented to acquaint the participants with testing procedure. Following familiarization, perturbations were triggered at 16.75 m/s2 with a displacement of 20 cm.
2.4 Data collection and analysis
An eight camera motion capture system with a sampling rate of 120 Hz recorded full body kinematics (Motion Analysis Corporation, Santa Rosa, CA). The Helen Hayes marker set with 29 markers placed on bilateral bony landmarks, head and trunk was used to compute the joint centers and center of mass (COM) [8]. An additional marker was placed on the treadmill belt to identify the instant of perturbation onset. The raw marker data were low pass filtered using the fourth order Butterworth filter with a cut off frequency of 6Hz. The kinematic variables were computed using custom written algorithms in MATLAB version 2014b (The MathWorks Inc, Nactick, MA).
Outcome: The center of mass (COM) position was recorded relative to the anterior margin of the base of support (BOS) at touchdown of the stepping limb in the anteroposterior direction (XCOM/BOS) and normalized to the individual’s foot length. A more positive XCOM/ BOS would indicate greater instability in the forward direction. The XCOM/BOSwas recorded touchdown as it would represent the individual’s stability at the instance when they re-establish BOS after the initial balance loss at liftoff.
2.5 Gait assessment
Gait parameters were recorded using an electronic mat GaitRite (CIR Systems, Inc., Sparta, NJ). It consists of sensors embedded into 12 x 2 feet mat which measures spatial and temporal gait parameters via the accompanying GaitRite software (GaitRite Gold, Version 3.2). To record the steady state walking pattern, subjects began walking about 1 meter before stepping on the mat and continued walking about 2 meters beyond the mat (Figure 1).
Outcome: Gait velocity was measured while the subjects walked on the mat and was defined as the distance walked in the walking time for that specific trial [9]. Gait velocity was used as the outcome measure as the effect of dual-tasking is reflected consistently on gait speed across studies in healthy adults [9,10] and in stroke survivors [5].
2.6 Cognitive task
Subjects performed a mental arithmetic or serial subtraction task involving counting backwards from a specific two-digit number by a given single-digit number. The number of correct responses over a period of 30s were recorded while standing (single-task) and while performing the balance tasks (dual-task).
2.7 Experimental protocol
Subjects first received standardized instructions on how to perform the cognitive task followed by one familiarization trial. Subjects then performed a single trial of the cognitive task in standing position. This was by followed by random allocation of the subjects to evaluate dual tasking function on one of the three balance tasks i.e. either the LOS, gait or reactive balance tasks. Within each of the groups the balance task was performed in single-task i.e. performing the balance task without the cognitive task and dual-task conditions. The duration of each trial was 10-30s depending upon type of balance task.
- Limits of Stability (LOS): After a familiarization trial, the subjects performed the LOS task in isolation followed by performing the LOS tasks concurrently with the serial subtraction task. In the dual-task condition, subjects initiated the balance and serial subtraction task simultaneously upon hearing the auditory cue.
- Gait: Subjects initially walked for three trials at preferred walking speed followed by another block of three trials in the dual-task condition wherein the subjects began the serial subtraction walking tasks simultaneously.
- Reactive balance task (RB): Upon familiarization to this balance task, subjects were exposed to a single trip in absence of a cognitive task (single-task). Subjects then performed the balance task in dual-task condition. They were instructed to begin the cognitive task upon hearing a verbal prompt. The cognitive task and perturbation trial were initiated simultaneously. The a triplike perturbation was then triggered about 10-15s after initiating the trial.
For the LOS and reactive balance tasks, half of the subject performed balance tasks in the single-task condition prior to the dual-task and the other half of the subjects performed trials in the reverse order.
2.8 Motor cost
The effect of dual-tasking on both balance and cognitive parameters was assessed by comparing the absolute values for all balance, gait and cognitive variables between single- and dual-task conditions. To compare the effect of dual-tasking across the balance tasks between the three groups, the motor and cognitive dual-task cost was measured using following the formula [11].
[(Single-task — Dual-task)/Single-task]*100
In this study, the motor cost represented the performance on the balance measures in dual-task condition and the motor cost was calculated for each of the balance tasks. Similarly, cognitive cost represented the performance on the cognitive task in dual-task condition while performing each of the three balance tasks. Thus, positive value for motor and/or cognitive cost reduced performance on the individual balance task and/or cognitive task under dual-task condition where as a negative value for motor and/or cognitive cost indicated improved performance in the dual-task condition on the respective motor/cognitive task. The differential challenge of the balance tasks was determined based upon the motor cost under the respective dual-task conditions.
2.9 Statistical analysis
To analyze the effect of dual-tasking across the different balance tasks, between the groups, 3 x 3 two-way repeated measures ANOVA was performed for motor and cognitive costs with the balance tasks (reactive balance, gait and LOS) as within-groups factor and groups (young, age-match adults and stroke) as the between group factor. The significant interactions and main effects were resolved by independent t-tests. Further, independent t-tests were performed to examine the difference between motor and cognitive costs for each balance task within each group. The alpha level was set at p < 0.05. All the analyses were performed using SPSS 24.00 (IBM. Inc).
3. Results
3.1 Effect of dual-tasking on balance tasks
The effect of dual-tasking on balance measures was compared by calculating the motor costs. There was a significant interaction between the type of balance task and the groups for motor cost [F (2,62) = 2.66, p <0.05, Figure 2a). The effect of dual-tasking during different balance tasks was not the same for each of the groups.Within the groups the motor costs differed across the balance tasks [main effect of balance task, F (2,31) = 3.02, p = 0.05]. For the young adults, the motor cost for the LOS tasks was significantly lower as compared with the other two tasks (p < 0.05 for LOS vs. RB and for p < 0.01 LOS vs. gait). Similarly, among the age-matched adults, the motor cost tended to be lower for LOS tasks as compared with the other two tasks (p < 0.05 for LOS vs. RB and LOS vs. gait). Within the stroke group, although there was a trend towards higher motor cost during the LOS task as compared with the other two tasks, there was no significant difference in motor cost between the three tasks (p > 0.05 for all comparisons).
There was a significant main effect of group for the motor cost, [F (2,31) = 14.89 p = 0.00, Figure 2a]. For the RB task, the motor cost was significantly greater for the age-matched and stroke groups than that for young adults (p = 0.01 for age-match vs. young, p = 0.01 for stroke vs. young) however, there was no difference in motor cost between the age-matched adults and stroke survivors (p > 0.05). Similarly, for gait task the motor cost was significantly lower in younger adults than the other two groups (p = 0.02 for young vs. age-matched and p = 0.04 for young vs. stroke). There was no difference in motor cost for gait between stroke and age-matched adults (p > 0.05). For the LOS task, the motor cost was significantly different between all three groups – young adults< age-matched adults (p = 0.00), age-matched adults< stroke survivors (p = 0.00) and young adults< stroke survivors (p = 0.00).
With regards to the cognitive cost, which indicated the performance on the cognitive task in dual-task conditions, there was no significant interaction between the type of balance tasks and the groups [F (2, 31) = 1.56, p > 0.05]. Further, there was no significant main effect of balance tasks on the cognitive costs [F (2,62) = 1.90, p > 0.05]. However, a a significant main effect of group was observed on the cognitive cost of dual-tasking[F (2,31) = 3.58, p < 0.05, Figure 2b]. The cognitive cost for RB task did not differ between the groups (p > 0.05 for all comparisons). The cognitive cost for gait task was greater among age-matched adults and stroke survivors as compared with younger adults (age-matched and stroke > young adults, p < 0.05). For the LOS task as well, the cognitive cost was higher in stroke survivors and age-match adults than younger adults (age-matched and stroke > young adults, p < 0.05) with no significant difference in cognitive cost between age-matched and stroke survivors (p > 0.05).
Further, a comparison of motor and cognitive costs for each of the tasks among all groups showed significantly greater cognitive cost for reactive balance task (p = 0.03) and LOS (p = 0.00) tasks among the younger adults with no significant difference between the costs for gait task (p > 0.05) (Figure 3a-c). The age-matched adults and stroke survivors showed a higher cognitive cost for LOS (p = 0.00 for agematch, p = 0.01 for stroke) however there was no difference between motor and cognitive costs for the RB and gait tasks (Figures 3d-i).
4. Discussion
This study aimed to examine the whether the CMI pattern differed with regards to the type of balance task and effect of aging with and without a stroke on CMI pattern across the motor tasks. As hypothesized, the type of balance task influenced the CMI pattern.
Further, chronic stroke had some effect on the CMI pattern. Generally, the age-matched adults and chronic stroke survivors showed higher motor costs for all three tasks however, marked differences between all three groups were observed predominantly for the LOS task. A decline in cognitive performance was also observed among all the groups during dual-tasking.
4.1 CMI across balance tasks in healthy nervous system
The effect of dual-tasking differed between the motor tasks in both younger and older adults, such that the motor cost for RB and gait tasks were similar and greater than the LOS task. The differences in motor costs may arise from the neural processes involved in movement control and bio-mechanical demands of the tasks itself. The supplementary motor area, which is involved in movement planning and posture control is proposed to have a central role in intentional, self-initiated movements, as that observed during the LOS task [12]. Although gait and reactive balance tasks could be controlled by the central pattern generators in brainstem and spinal cord, a descending influence of cortical centers via cerebellum facilitates modulation of these postural tasks [13-15]. Further, walking and compensatory stepping tasks require coordinated movements between trunk and lower extremities to maintain balance over changing BOS. On the contrary, a voluntary reaching task such as LOS, involves controlling the upper body segment and ankle torque while maintaining balance on a constant BOS. Considering the involvement of cortical areas during a bio-mechanically less challenging voluntary task, it is possible that individuals could explicitly allocate attention to maintain stability on the LOS task. The neural processes modulating tasks that pose greater threat to stability are likely interfered with greater extent by concurrent working memory tasks resulting in higher motor costs. These findings are in line with a previous study that showed the attentional demands of postural tasks progressively increased from sitting, to standing, to walking observed by an increase in reaction time on an auditory task [2].
Dual-tasking often results in a trade-off of attentional resources between motor and cognitive tasks, and it is possible to minimize the cost of performing either of the tasks in the presence of explicit instruction [16,17]. We observed implicit prioritization of motor tasks during RB and LOS, as the cognitive cost was considerably higher than motor cost for these tasks (see figures 3a & 3c). It suggests that younger adults attempted to trade off the performance on the cognitive task to achieve stability during momentary balance loss whether voluntary (LOS) or involuntary (RB). During the gait task however, the two costs were similar indicating a mutual cognitive-motor interference (figure 3b). Considering that walking is a continuous task, individuals likely attempt to allocate equal attentional resources to both motor and working memory tasks affecting performance on both the tasks equally.
4.2 Effect of aging on CMI across balance tasks
Similar to young adults, age-matched older adults also showed modulation in attentional demands with higher motor cost during less stable RB and gait tasks than during the LOS task. However, the effect of aging was evident by higher motor costs for all balance tasks and higher cognitive cost for the gait and LOS tasks [18]. With the normal aging process, there is a decline in balance control due to reduced proprioceptive function, impaired synergistic contraction of muscles contributing to greater co-activation of muscles and delayed postural reflexes [19-21] as well as reduced cognitive functions like working memory and information processing speed [22-24], the cognitive functions deemed crucial for balance control [25]. Considering reduced central capacity with aging alongside declining neuromuscular function could possibly limit greater allocation of attentional resources to motor tasks, contributing to higher motor costs as seen in our study [26]. Unlike young adults, age-matched adults prioritized the motor task only during the LOS task (Figure 3f) as seen in previous studies [27]. They were unable to do so during the RB task, which in fact posed higher threat to balance than the LOS task. The decline in central capacity with aging also appears to interfere with the ability to prioritize the motor task when the relative demands of the balance task are greater.
4.3 Effect of stroke on CMI across balance tasks
Unlike healthy adults (younger and older), the stroke survivors failed to show a specific trend for the motor cost. The motor costs were similar for all three tasks within this group. Stroke survivors perhaps demonstrate a disproportionate ability to divide attention between motor and cognitive tasks compared with healthy age-matched controls. Although it is well-known that there is a decline in dualtask function post-stroke [28-33], most studies have reported CMI during a single motor task such as gait, quiet standing, or voluntary stepping. Furthermore, no study thus far has examined CMI during compensatory stepping from large perturbations which is impaired in this population [34,35]. Hyndman et al. (2006) compared the CMI during quiet standing and walking and observed that a concurrent cognitive task affected on walking speed but not anteroposterior way during standing, suggesting that a simple task like standing may not be affected by a cognitive task among chronic stroke survivors [36]. Similarly, we observed a decline in both motor and cognitive performance on all the motor tasks focused on dynamic balance. The fact that all dynamic balance tasks incurred equal attentional resources regardless of the differential postural challenge, it is possible that the ability to flexibly allocate attention to postural task remains affected in chronic phases of stroke.
In comparison with age-matched adults, the motor cost was greater for the LOS task whereas the costs were comparable between the groups for gait and RB task.As stroke affects higher cortical areas which predominantly influence movement control during voluntary balance tasks, a cortical injury during stroke could possibly affect the ability to allocate attention to carry out voluntary tasks under cognitive loads. This may explain a higher motor cost for the voluntary, LOS task in stroke survivors as compared with the age-matched controls despite implicit prioritization of the motor task (see figure 3i) [27]. Considering that the stroke survivors in this study were in chronic phase of recovery (at least 3 years post stroke), it may be difficult to conclude that the CMI observed in this population may be directly due the stroke-related pathology [5].
The similarities in motor and cognitive costs between age-match controls and chronic stroke survivors during RB and gait tasks could be potentially related to motor recovery and continued community ambulation in the chronic phase post-stroke. During community ambulation, the demand for dual-tasking ability is significant. Independent mobility in different community settings (e.g. grocery stores, hospitals, school, post office, gym etc.) may perhaps facilitate gains in balance or assist in developing compensatory strategies. The severity of motor impairment is negatively associated with community mobility and balance [37]. The stroke survivors in our study showed only mild to moderate levels of motor impairment (CMSA leg score 3-6/7), were community ambulators and were involved in light to moderate levels of physical activity on daily basis (RAPA1 score 4-6/7). This could possibly explain similar dual-task function in stroke survivors and age-matched healthy controls. Very few studies have investigated the CMI in chronic stroke survivors with their healthy counterparts. Two studies compared effect of dual-tasking in ambulatory older stroke survivors with > 3 years post stroke with healthy controls [5,38]. These studies also reported a similar decline in gait velocity and cadence in older chronic stroke survivors and healthy adults in dual-task condition suggesting that dual-task deficits in chronic stroke survivors may be, in part, due to aging.
Dual-tasking is increasingly incorporated in clinical settings for both assessment and training. At the rehabilitation level, our findings emphasize the need of testing cognitive-motor interference during varied postural tasks among people aging with and without a cerebral injury from stroke. Consequently, the dual-task training should incorporate specific balance tasks which individuals find more challenging for example stepping or walking. Given some degree of similarity in CMI between older healthy individuals and stroke survivors, it is possible that stroke survivors show similar gains in dual-tasking ability with training. This study extends the literature related to CMI by demonstrating the attentional demands of balance tasks that may pose differential threat to stability among healthy younger adult and adults aging with and without a stroke. Although in the chronic stage, stroke survivors may show similar CMI as their healthy counterparts for dynamic balance tasks such as compensatory stepping, quasi-static balance tasks requiring constant postural movement such as leaning forward may be affected differently by cognitive interference in this population as compared with age matched adults.
Competing Interests
The authors declare that they have no competing interests.
Abbreviations
ANOVA = Analysis of variance BOS = Base of support CMI = Cognitive-motor interference COM = Center of mass CoP = Center of pressure LOS = Limits of stability MXE = Maximum center of pressure excursion RB = Reactive balance XCOM/BOS = Center of mass position relative to base of support