1. Introduction
High frequency oscillatory ventilation (HFOV) is considered an advanced, unconventional, and presumably protective mode of mechanical ventilation used in selected cases of pediatric respiratory failure. By using HFOV, we recruit diseased lung and improve oxygenation, and to some degree ventilation, by constantly distension using high mean airway pressure (MAP) with tidal volume less than anatomic dead space. Several studies in medical literature have suggested more improvement, mainly in oxygenation and also in ventilation, in patients managed with HFOV when compared to conventional mechanical ventilation (CMV) [1-5]. Despite the wide use in all age groups and many success stories with this mode of HFOV, some newly published studies have described contradicting results. Recently, in two randomized, controlled, multicenter trials in adults, the use of HFOV was of questionable value and not associated with decreased mortality [6,7]. Even in the pediatric age group, some new studies have shown no clear benefit over continuous mandatory ventilation (CMV) and even shows the tendency toward increased mortality and complications [8-10].
It is not clear thus far which factors contribute to the success or failure of the HFOV mode of ventilation. Several factors might contribute to the failure of HFOV: (1) underlying disease, (2) pathophysiology and disease nature, (3) degree and type of gas exchange impairment, (4) duration of CMV, and/or (5) maximum settings of previous conventional ventilation.
No standardized international practice guidelines are available for the proper indications for switching from conventional mode to HFOV, which cases will improve better on HFOV, and proper adjustment of the HFOV settings. Therefore, the objectives of this study were to identify which patient profiles could be best treated with HFOV in medical centers, especially with the lack of readily available extracorporeal membranous oxygenation (ECMO) rescue therapy, which ones will not improve or even worsen the condition, and the best possible way of selecting and adjusting HFOV settings in the first 48 h.
2. Materials and Methods
A retrospective observational study was conducted in the pediatric intensive care unit (PICU) at King Fahad Medical City (KFMC), a tertiary care hospital located in Riyadh, the capital of Saudi Arabia, between September 2010 and December 2017. All patients under 18 years of age with respiratory failure who were on CMV and then switched to HFOV were eligible to participate in this study. Patients whom were directly put on HFOV without previously being on CMV. Patients with incomplete electronic charts were excluded.
All included patients were ventilated using 3100-A HFOV machine (SensorMedics, Yorba Linda, California). Data were obtained from patients’ medical records using an electronic chart viewer and collected on specially designed capture sheets by two pediatric intensivists working in the unit as the primary investigators. Collected data included patient’s demographic data, hospital and PICU admission dates, background diseases if any, underlying cause of respiratory failure and its pathophysiology (diffuse alveolar disease [DAD], small airway disease [SAD], air leak, mixed pathology, and others), duration of CMV and HFOV, reasons for switching to HFOV, and survival at 48 h. Ventilator settings, blood gases, oxygen saturation index (OSI), blood pressure (BP), and vasoactive-inotropic score (VIS) were all extracted just before connecting the patient to HFOV and 0, 2, 6, 12, 24, and 48 h after connecting the patient to HFOV. In children, VIS is a reliable marker of cardiovascular support and provide additive value to existing pediatric acuity scores in this population [11].
Primary outcome was improvement versus worsening of OSI, pH, PCO2, and vasoactive score. Secondary outcome was survival rate at 48 h. The pathophysiology of the respiratory disease was determined based on the diagnoses as documented in the patient’s medical record by the treating medical team. The diagnosis was confirmed by evaluating collected blood gases values, support provided on ventilator, and chest radiographic findings that were reviewed by the primary investigators and supported by the official radiology report. Oxygenation failure was defined as persistent desaturation below 90% while on 100% fraction of inspired oxygen (FiO2) or when there is a need for at least 80% FiO2 to maintain an O2 saturation above 90% or the need for more than 60% FiO2 to maintain an O2 saturation of more than 84%. Ventilation failure was defined as pH less than 7.30 with elevated CO2 compared to baseline. High settings were defined as peak inspiratory pressure (PIP) greater than 30 cm H2O. Hypotension was defined as systolic BP readings below the 5th centile for age.
2.1 Ethical considerations
The study protocol was approved by KFMC institutional review board (IRB), and collected data were treated with strict confidentiality as per KFMC institutional regulations.
2.2 Data analysis
Continuous data were presented as mean and standard deviation (SD) if normally distributed or median and interquartile range if the distribution was not normal. Categorical data were presented using frequencies and percentages. Continuous variables were compared using the independent-sample t-test or the Mann-Whitney U-test. Categorical variables were compared using the Fisher’s exact or Pearson’s chi-square test.
Differences were considered statistically significant when two-tailed P-values were less than 0.05. Calculations were performed using SPSS Statistics, version 22.0 (SPSS Inc., Chicago, IL., USA).
3. Results
With a study period of over seven years, it was found that HFOV was used to support respiratory failure 241 times. Twenty-eight cases out of the 241 were excluded due to missing and important data in the medical records. Two patients were treated directly with HFOV without any conventional ventilation, and four patients were switched to HFOV from either the continuous positive airway pressure (CPAP) or non-invasive ventilation (NIV) modes; these cases were also excluded from the analysis. Two-hundred seven HFOV episodes were analyzed.
3.1 Patient characteristics
The median age of our cohort was 12 (interquartile range [IQR] 4-48) months with 52.7% male cases. The most common cause of respiratory failure that required HFOV rescue use was acute respiratory distress syndrome (ARDS) in 100 (48.3%) cases followed by pneumonia in 34 (16.4%) cases. Cases with small airway disease (SAD) represented only 4.9% of the studied cohort with cases of asthma and eight with bronchiolitis diagnoses. Cases with mixed pathophysiology of diffuse alveolar disease (DAD) with SAD accounted for 15% of the cases. A total of 32 out of the 207 cases had significant respiratory failure that required HFOV but did not fit into any of the commonly described indications for acute respiratory failure that usually benefit from HFOV. The majority (87.4%) of the study patients had an underlying chronic illness, and the most frequently observed illnesses were malignancies (hematological and solid organ), neurological diseases, and immunodeficiency disorders (15.5%, 13%, and 12.6%, respectively). Chronic lung disease was only encountered in 14 cases (6.8%).
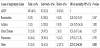
Oxygenation failure was the most frequently reported reason for shifting to HFOV followed by ventilation failure and then toxic high ventilatory settings, (77.3%, 68.6%, and 56% respectively) (Table 1). Most of our pre-HFOV cases were moderately to severely hypoxemic with a median OSI of 17.8 (IQR 14.3-22), acidotic with median pH pre-HFOV of 7.18 (IQR 7.06-7.28), and hypercarbic with median PCO2 pre-HFOV of 69 (54, 93 IQR). Inhaled nitric oxide (iNO) was tried before HFOV only in 24.6% of the cases, and a neuromuscular blocking agent was used before the transition in more than half of the cases (54.6%). Hypotension while on conventional ventilation just before switching to HFOV was encountered in 19.3% of the cases and increased by almost two-fold during the first 48 h of commencing HFOV therapy (36.4%).
3.2 Outcome
Over the first 48 h period after transition to rescue HFOV, a significant improvement in oxygenation parameters were observed in our cohort using this modality of ventilation associated with the need for gradual increment in mean airway pressure (Figure 1). The target of less than 60% FiO2 was achieved in many of the cases within 24 h, but the response was weaker on the second day.
The same improving trend in ventilation and acid base status was observed but was faster with the target being achieved within 6 to 12 h (Figure 2). The change was associated with an increment of HFOV amplitude but more significant titration of frequency of oscillation was observed in our group (p <0.05).
3.2 Short term (48 h) mortality risk
Table 2 shows patients’ demographics and variables related to illness severity, underlying conditions, pathophysiology, and its possible impact on short-term survival. The overall 48 h survival of HFOV rescue therapy was achieved in 82.2% of the cases. No significant differences between patients who survived 48 hand the non-survivors in terms of age, weight, height, gender, presence of underlying diagnosis, prior to the use of iNO, O2 OSI, and pre-HFOV P-CO2 were noted (p >0.05).
The lower chance of short-term survival was associated with shorter duration of CMV, lower pH, not using neuromuscular blocking agents, presence of sepsis, high vasopressor index, and hypotensive status just before switching to the rescue therapy or within 48 h of initiation of HFOV therapy (p <0.05).
Table 3 shows the 48-hmortality based on different groups of underlying diseases. The only significant association in our cohort was found with patients with hematological malignancies (OR 8.73, 95% CI 1.58-48.06; p = 0.013). Patients with underlying cardiac disease or immunodeficiencies had higher mortality rates compared to normal (control) patients, but this difference did not reach statistical significance. The short term 48-h survival in patients who were rescued for ARDS using HFOV was 82%. When using ARDS as a reference group, the only statistically significant difference in mortality was seen in the poorly identified group that did not fit into either the DAD or SAD categories (Table 4).
The 48 h survival rates in cases of oxygenation and/or ventilation failures and high settings were 83.2%, 79.7%, and 83.2% respectively (p= 0.016). CMV settings showed no significant difference between surviving versus non-surviving groups (Table 5).
4. Discussion
As the debate about the value of HFOV in pediatric respiratory failure refractory to conventional means continues, the greater the need to share different experiences from different areas of the world to try to add to our understanding of this unresolved issue. We were able to demonstrate the clear value of HFOV rescue therapy in improving both oxygenation and ventilation as has been described by others from different areas of the world in our study population [1-5]. The majority of our patients had DAD as a pathophysiology, which explained the failure of both oxygenation and ventilation. HFOV was also used in our group for small numbers of SAD, which was very successful after being refractory to conventional ventilation strategies. Patients' demographics and CMV settings did not have significant impact on short term survival in our cohort.
In our patients, a remarkable association of number of days on CMV with the patient outcome was noted as patients who died within 48 h had in average a shorter duration of CMV (p= 0.046). The other factors that had an impact on 48-hsurvival were the last pH reading, presence of sepsis in the diagnoses, and hypotension just prior or during the 48 h of HFOV use (p <0.01). The use of pre-HFOV neuromuscular blocking agents was observed more with the survivors (p <0.001) and also with the trial of inhaled nitric oxide, but it did not reach statistical significance in the latter (p= 0.095).
Our results showed a higher mortality rate in patients with underlying hematological malignancies and to a lesser degree, patients with cardiac problems and immunodeficiencies. Based on the cause of respiratory failure and after comparing different categories with ARDS group, we were able to show an association of worse short-term outcome in the group that had respiratory failure but did not fit clinically or radiologically the findings in DAD or SAD, the commonly described indications for HFOV (OR of mortality 2.73, 1.13-6.58); p= 0.030).
In a recent publication, Retting et al. described an updated multicenter experience using HFOV in pediatric acute lung injury [12]. They emphasized in their publication, based on results from different centers from North America and Europe, the importance of underlying conditions and severity of hypoxemia, as measured by oxygenation index (OI) for predicting survival. For their patients, an immunocompromised state followed by cyanotic heart disease and then chronic lung disease were the cases that had the highest probability of death. Similarly, in our study we observed almost same pattern but to a lesser degree even though we are only addressing short-term mortality. Moreover, our study showed a higher mortality in patient with hematological malignancies. The higher mortality when using HFOV for refractory hypoxemia associated with malignancies and in post-stem cell transplant cases has been described by more than one recent publication [13,14]. These cases deserve the highest degree of vigilance and need additional well-designed trials to account for all possible confounding variables. We could not duplicate the findings of worse associated outcomes as reported by Retting et al. or the severity of hypoxemia as measured by OI. An interesting finding in our cohort was the association of short-term outcome with the hemodynamic variables. The significant association of lower pH value (not related to pCO2), hypotension before and during the HFOV trial, higher vasopressor scores, and presence of sepsis as secondary diagnosis with mortality is something to be further examined. The use of HFOV and its potential associated impact on cardiac function and hemodynamic instability especially in dehydrated patient is not new to medical literature [15,16]. This fact deserves special consideration when choosing this therapy modality after considering evolving evidence and the “hot” discussion questioning the value of HFOV when compared with more proper applications of CMV [8-10]. We believe in the value of using HFOV in our hospital, especially with the lack of ECMO support for the time being, but the burning questions will always be “Who will benefit the most?” and “How do we apply this mode of ventilation properly?”.
One more observation we noticed in our cohort after looking at the adjustment of the ventilator settings with high CO2. It was obvious from the data that we have been turning the frequency down more than increasing the amplitude. This practice is probably suboptimal and different from what is currently being recommended and practiced by other intensivists utilizing higher Hz and higher delta P strategies to protect the lung from high tidal volume injury.
This study is the first to address these points in the kingdom of Saudi Arabia, and hopefully it will help create unified guidelines for HFOV use and standardize PICU practices in this regard.
The retrospective design and data collection difficulties due to suboptimal archiving and the transition period to electronic health record were the major limitations of our study. Blood gases were mainly obtained from capillaries; thus, oxygenation index could not be used, and the surrogate OSI was utilized in our cases as it is proved by recent publication to be an acceptable alternative (as discussed in recent publications) to measure the severity of hypoxemia and is being used as an alternative measurement in the most recent definition of pediatric ARDS [17]. Another limitation of our study is the lack of detailed data on the 32 out of the 207 cases that had significant respiratory failure and were placed on HFOV. These cases did not fit into the DAD or SAD categories, which are the most frequently studied indications of acute respiratory failure that are managed by HFOV. These cases are worthy of analyzing as they presented a higher mortality risk, but this was beyond the scope of our current study.
Despite these limitations, we believe our study adds some insight about some of the factors that can be associated with short term improvement and mortality. These factors need to be taken into consideration when we choose to apply the HFOV modality as rescue therapy. Choosing the right patient, the right time, and the right technique will surely help us provide more convincing evidence of the value of this mode of ventilation. Having a good randomized clinical trial addressing these variables will be of great help to the pediatric physicians working in the intensive care to guide them to make the right choices for their patients.
5. Conclusion
Our study supports the great value of HFOV in improving oxygenation and ventilation when the conventional mode fails to achieve its goals. The underlying disease seems to have a significant impact on the outcome. In our population, we were able to confirm some of the already described known risk factors for mortality and unable to replicate others. Hemodynamic instability and sepsis are major contributors to worse outcomes and should be tackled vigilantly. We still need to learn more about the proper selection of patients, time of switching to HFOV, and the proper safe technique to apply HFOV in pediatric respiratory failure.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Rehab Gabr did the research proposal, collected the data, refined it,
assisted in data analysis and wrote the manuscript draft.
Mohammed Aboo helped in data collection, refining it, assisted in
writing the manuscript.
Jihad Zahraa helped refining the proposal, refining the data,
data analysis and in finalizing the manuscript. All authors read and
approved the final manuscript.
All had given the permission for submission.
Acknowledgments
We thank Ms. Michele Mella our department secretary for her help in entering the patient data collected into the electronic format. We thank Mr. Tariq Wani for his assistance performing the statistical analysis of our data.
Abbreviations
HFOV: High frequency oscillatory ventilation, PICU: Pediatric Intensive Care Unit, ARDS: Acute Respiratory Distress Syndrome, FiO2: Fraction of inspired Oxygen, DAD: Diffuse alveolar disease, SAD: Small airway disease, MAP: Mean airway pressure, CMV: Conventional mechanical ventilation, ECMO: Extracorporeal membranous oxygenation, KFMC: King Fahad Medical City, OSI: Oxygen saturation index, VIS: Vasoactive-Inotropic Score, PIP: Peak inspiratory pressure, IRB: Institutional review board, CPAP: Continuous positive airway pressure, NIV: Non-invasive ventilation, iNO: Inhaled nitric oxide, NMB: Neuromuscular blocking agent, OI: Oxygenation index