1. Introduction
Copper-bearing ore containing talc is regarded as the most refractory type in various chalcopyrite ores, which is predominantly due to the fact that it includes talc [1,2]. Talc is a gangue mineral in many base metal ores including sulfide ores [3]. Talc is a non-polar layered silicate mineral that is hydrophobic, so it is easily floatable [3]. Chalcopyrite is one of the sulfide minerals with good floatability [4-6]. When copper-bearing ores contain more talc, it is difficult to separate chalcopyrite and talc by conventional collectors such as xanthates, resulting in a low grade of copper concentrate. The low-grade copper concentrate causes heavy load in the ore transportation and smelting. In addition, Friedrich Mohs focused on selecting the minerals with his scale-talc, gypsum, calcite, fluorite, apatite, orthoclase, quartz, topaz, corundum, and diamond, where Mohs' hardness scale of talc is 1 [7,8]. That is to say, talc is the world's lowest-hardness ore. Therefore, chalcopyrite ore containing talc will be over crushed and over grinded, resulting in argillization to affect the flotation response [9]. The fine size talc adheres to the bubble surface, leading to sticky flotation bubbles, poor fluidity and poor selectivity. It adversely affects the subsequent flotation of copper-bearing minerals and the copper grade of concentrate. Talc not only deteriorates the flotation conditions, but also increases the consumption of flotation agents [1]. Therefore, it is necessary to develop novel separation processes easy to industrialize for copper-bearing sulfide minerals and talc.
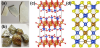
Figure 1 (a) and (c) show the common talc natural crystal and structure model, respectively. Talc [Mg3(Si2O5)2(OH)2] has a T-O-T (2:1 structure), including an octahedral brucite O-layer sandwiched between 2 silica T-layers [3]. Weak van der Waals forces are used to connect T-O-T-layers [3,10]. Through breaking talc particles, it forms two different types of surfaces-the basal plane (orface), and the edge [11]. The basal plane results from the cleavage of the weak bonding between silica layers, regarded to be electrostatically uncharged in aqueous solutions [11]. The subject showed that the negative charge can be imparted to the basal plane of talc by low levels of substituting SibyAl and Ti [12,13]. The basal planes are hydrophobic due to the low level of substitution and weak bonds exposed to the surrounding water. Wettability studies on the basal planes of talc reported contact angle values were in the range of 60-90° [14,15]. Besides, the edge forms from the rupture of strong ionic and covalent bonds of the silica and brucite layers [11]. Actually, the edges, electrostatically charged with high polarity, are hydrophilic [16,17]. Many researchers have used the density functional theory (DFT) to study properties of talc crystals such as geometric and electronic structure [18-25]. In the tetragonal group (space group I42d), chalcopyrite crystallizes with four formulas (CuFeS2) per unit cell [26]. Figure 1(b) and (d) show the natural crystal and structural model of the most common chalcopyrite. The lattice parameters were a=b=5.289 Å and c=10.423 Å; the Fe-S and Cu-S bond lengths were 2.257 and 2.302 Å, respectively [26]. In the solid structure, the oxidation number of iron, copper, and sulfur is +3, +1, and -2, respectively [26]. At 0 K, chalcopyrite is assumed to be an anti ferromagnetic material with alternate planes of iron with spin up or down along the c direction [27]. Some previous researchers carried out DFT calculations on the chalcopyrite's bulk and surface structure to achieve geometric structures in good agreement with experiments [27-35]. They also studied the geometric and electronic properties of chalcopyrite [27-33]. In a word, although talc and chalcopyrite have good floatability, their crystal structures are different, which provides the possibility of suing selective depressors for their flotation separation.
With more and more attention being given to copper mineral recovery, a review of separation technology and selective depressing agent literature is proposed based on some recent findings. This work attempts to provide an explanation for the current state and use of selective depressing agent. It is expected that this knowledge will expedite the development of efficient, industriable, operable separation of chalcopyrite and talc.
2. Separation Technologies
The following four methods are used for the separation of copper sulfide minerals and talc [1,6,9]: (1) Gravity concentrating in advance to remove talc and then recovering copper sulfide minerals by flotation; (2) Preferential talc flotation followed by copper sulfide mineral flotation; (3) Adding depressors for talc with the flotation of copper sulfide minerals; (4) Bulk flotation of talc and chalcopyrite. Wherein, removing talc in advance by gravity concentration leads to reduced copper recovery, thus it is rarely used.
Process for depressing talc with flotation of copper minerals [1,9]: Efficient depressor for talc is used, and then collector is added for the flotation of chalcopyrite. However, some talc can easily enter the concentrate by foam entrainment, resulting in a decrease in chalcopyrite grade.
Bulk flotation of talc and chalcopyrite [1,9]: Bulk flotation refers to the flotation of talc and chalcopyrite to concentrate without pretreatment, then acid leaching can be used to remove talc from concentrate. This method can cause the circulation of talc during the process and the deterioration of flotation, which increases the complexity of the process.
Preferential flotation of talc before the flotation of chalcopyrite [1]: Using frothing agents with good selectivity to float some parts of talc before the flotation of sulfide minerals can reduce the interference of talc to the chalcopyrite flotation.
3. Selective Depressing Agents
In the flotation process, the depressor adsorbs on the mineral surface to form a hydrophilic film to prevent the collector from interacting with non-target minerals [36]. According to previous studies, the main depressors for talc can be classified into several categories (Table 1).
3.1 Carboxymethyl cellulose (CMC)
3.1.1 Molecular structure and properties of CMC
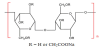
CMC Carboxymethyl cellulose is a cellulose derivative whose skeleton consists of glucopyranose polymer, often used as the sodium salt (Figure 2) [39]. Some of its hydroxyls are substituted by carboxymethyl groups [39]. CMC’s degree of substitution, DS, is one of the most important features. It affects the CMC molecule solubility as well as the solution characteristics [59]. The free acid strength of CMC is similar to that of acetic acid, with the ionization constant of 5×10-5. Therefore, the form of carboxyl in CMC is related to the pH value, and exists in the form of -COO- under alkaline conditions and -COOH under acidic conditions [37].
3.1.2 Depression mechanism of CMC on talc
The research on adsorption of CMC on talc in the pH range of 2-11 showed the CMC can decrease negative zeta potential of talc with a shift of the IEP from pH 2.5 to 3.5 [60,61]. Moreover, the adsorption of CMC on talc was affected by changes of pH and ionic strength. These results show the electrostatic force plays an important role in the adsorption of CMC on talc. In general, the main driving forces for CMC adsorption on talc consist of electrostatic interaction and hydrogen bonding instead of hydrophobic force [60].
Wang and Somasundaran [60] found that it is significant for the changes in the infrared bands in the region of 1000-1080 cm-1, with the C-O stretch coupled to the C-C stretch and O-H deformation through infrared spectrum comparison before and after reaction of CMC and talc. So it supports strong hydrogen bonding of polysaccharides on the solid [38]. After CMC was adsorbed on talc, 1632 cm-1 and 1439 cm-1 absorption peaks were detected and the Mg-O bending vibration peak of 534 cm-1 in the original talc sample moved to 501 cm-1. It indicated -COO- in CMC chain has chemical reaction with talc surface. Luo et al. [38] pointed out that the adsorption of CMC to talc includes electrostatic force, hydrogen bonding and chemical bonding.
In addition, as one of the most widely used talc depressors, CMC has been studied as follows: the effects of solution pH and ionic strength on CMC depression or adsorption control [59,62], the effects of metal ions on CMC depressing talc [63-65], the effects of CMC substitution degree and molecular weight on its depression to talc [59,66-68].
3.2 Chitosan
3.2.1 Molecular structure and properties of chitosan
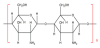
Chitosan, with hydroxyl groups and amino groups, is a natural cationic polysaccharide, which is one of the most abundant organic materials in nature [40,69-71]. It is a polysaccharide from the deacetylation of chitin. Figure 3 shows the schematic diagram of molecular structure of chitosan. The pKa value is 6.5 for the amino group of chitosan. In acidic pH range, chitosan is positively charged with the protonation of the amino group. It makes chitosan watersoluble, binding to negatively charged surfaces. However, as the pH increases above neutral, chitosan loses its charge, becoming sparingly soluble in water [72,73].
3.2.2 Depression mechanism of chitosan on talc
The depression of an adsorbed chitosan layer on flotation of talc is affected by a change of the solution PH [40,41]. With the chitosan positively charged at pH 3, its adsorption changed the zeta potential of talc from negative to positive [74,75]. However, with the insoluble chitosan on the surface of the talc at pH 9, the adsorption of the noncharged chitosan made the electric double layer slip surface outward. Therefore, it causes the surface potential to zero. The chitosan adsorbed on talc surfaces through physical interactions at pH 3 and 9. The chitosan, positively charged at pH 3, was adsorbed on the talc surface through hydrogen bonding. At pH 9, the chitosan, becoming sparingly soluble in water, deposited on the talc surfaces, leading to the increased adsorbed amount of chitosan [40,41]. It should be noted that chitosan also has a negative impact on chalcopyrite flotation [76].
3.3 Gums-guar gum, locust bean gum, etc
3.3.1 Structure, properties and depression mechanism of guar gum
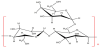
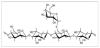
Guar gum, also called as Cyamopsis gum, Guaran, Guyan, Guarina or Glucotard, is a natural non-ionic, water soluble polysaccharide obtained from guar plant [6]. It is a galactomannan consisting of one galactose on every other mannose unit [77]. The ratio of mannose to galactose unit (M/G) is in the range from 1.5:1 to 2:1 because of climate variations [78]. Hydroxyl groups are contained in mannose and galactose-nine are accommodated into each guar monomer unit [42,79]. Figure 4 shows the molecular structure of guar gum.
Nonionic guar gum gives constant viscosity over the broad pH range. The practical stability is in the range of pH 4 to 10.5. Because of numerous hydroxyl groups across the chain, hydrogen bonding is formed in aqueous solution by guar gum. The basic mannose chain structure of galactomannan, with the single membered galactose branches, increases exposed hydroxyl groups [42].
Nonionic guar gum gives constant viscosity over the broad pH range. The practical stability is in the range of pH 4 to 10.5. Because of numerous hydroxyl groups across the chain, hydrogen bonding is formed in aqueous solution by guar gum. The basic mannose chain structure of galactomannan, with the single membered galactose branches, increases exposed hydroxyl groups [42].
Electrokinetic studies of adsorption of guar on talc at pH 2-11 showed that guar gum decreased the negative zeta potential of talc rather than reversing it. The changes in pH and the ionic strength level do not affect the adsorption of guar gum on talc. Thus, the electrostatic force is not the main force for the adsorption process. Pyrene fluorescence showed no hydrophobic domain formation for the adsorption of guar gum at talc-aqueous interfaces [43]. The neutral guar gum is adsorbed on the surface of minerals mainly by hydrogen bonding [9,11,44,45].
3.3.2 Structure and properties of locust bean gum and its depression mechanism
Locust bean gum is a vegetable gum from the seeds of the carob tree. It contains chiefly high-molecular-mass hydrocolloidal polysaccharides, which are composed of galactose and mannose units combined by glycosidic linkages (also described as galactomannan) [40,41,80,81]. Figure 5 shows the molecular structure of locust bean gum.
Zeta potential measurements were performed on chalcopyrite and talc before and after the addition of locust bean gum. After adding 2000 g/t locust bean gum, the magnitudes of the zeta potentials of both talc and chalcopyrite decreased. That is to say, locust bean gum adsorbed on the surfaces of both minerals. As locust bean gum is non-ionic, the shear plane of the electrical double layer could be stretched by its adsorption on the mineral surface further away from the surface, which lowers the magnitude of the zeta potential. Other studies showed the similar results-the adsorption of non-ionic agents reduced the negative zeta potentials of talc and chalcopyrite, but did not reverse the charges [40,41].
Currently, there are many natural gums with similar properties for depression of talc. Some researchers have tested some related depressors such as gum Arabic [46], sesbania gum [47], tragacanth gum [48] and galactomannan [49]. These depressors, basically similar in their mechanism of action, mainly differ in the pH range of effective depression. In production practice, the economic efficiency and flotation indexes should be considered for the optimal benefits [49,82].
3.4 Lignosulfonate
3.4.1 Structure and properties of lignosulfonate
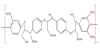
Lignosulfonate, a water-soluble lignin extracted from the sulfite pulping process, is lypo hydrophilic molecule because of hydrophobic aromatic structure and the presence of the hydrophilic sulfonate groups on its structure [52]. Figure 6 shows the molecular structure of sodium lignosulfonate, whose molecule is approximate to a spherical three-dimensional network structure composed of 50 phenylpropane units [83]. Macromolecule contains hydrophobic backbone (C6– C3 structure moieties linked together by ether and carbon bonds) branched with hydrophilic side chains (carboxyl, sulfonic, and phenolic hydroxyl groups). Therefore, it possesses a certain degree of surface activity as well as remarkable affinity for some metal ions [84]. The pH increased with the increase of the surface charge of lignosulfonate molecules and the aggregation degree of lignosulfonate in solution because of the ionization of sulfonic and phenolic hydroxyl groups [85].
3.4.2 Depression mechanism of lignosulfonate
With the increase of surface charge and degree of amnionicity of reagents, the adsorption density of lignosulfonates on talc decreases, showing that electrostatic forces largely control the adsorption process on the net-negatively charged talc surface [50]. Moreover, the infrared spectroscopy analysis showed that sodium lignosulfonate can be chemically adsorbed on the talc surface under strongly alkaline conditions, thus achieving selective depression of talc [51]. The effects of lignosulfonates on the floatability of talc is in alkaline media with various pH values ranging from 7 to 11.2 [52]. Depression of chalcopyrite flotation by lignosulfonates occurs at high pH with lime used for pH control. Therefore, calcium lignosulfonates are stronger depressors compared with sodium salts in lignosulfonate adsorption and mineral depression. However, calcium salts also appear to be less selective [86]. It is different for the adsorption mechanism of each lignosulfonate on the mineral surface. The actual mechanism may be hydrogen bonding, electrostatic interaction, chemical bonding and complexation of calcium ions on the mineral surface [53].
3.5 Al3+, Cr3+, Fe3+ and other metal ion depressors
The natural flotability (and zeta potential) of talc, as with other minerals, can be changed substantially through the adsorption of hydroxo complex species of Al3+, Cr3+, Fe3+, etc. [10]. In addition, Ca2+, Mg2+, Cu2+, and Pb2+ affect the species as well as hydrophilicity or hydrophobicity of talc surface [87,88]. Different metal ions have different effects on the flotation response and potential of talc. When these metal cations are added to the solution, the potential of talc surface changes obviously, indicating the adsorption of several metal ions on the talc surface. The pH values suitable for metal ion depressing talc are related to the favorable pH for metal hydroxide precipitation and the point of zero charge (PZC) of the precipitate [89].
Hydroxyl complexation ions are generated in the corresponding PH range of these above-mentioned metal cations. The adsorption of metal hydroxyl complex ions on talc surface is the main reason for the increase of talc surface potential [90]. There is a strong interaction between water molecules and the surface covered by metal hydroxide precipitation through hydrogen bonds. At the same time, due to the high polarity of metal hydroxide precipitation, the adhesive work of the system is higher than the cohesive work, which further inhibits the hydrophobicity of talc [91]. By using this property, talc can be depressed by adding metal ions in the process of flotation separation of chalcopyrite and talc.
3.6 Dextrin and modified starch
Starch is hydrolyzed to obtain dextrin, a product with low molecular weight and good water solubility. Dextrin is a non-ionic depressor, used to depress natural hydrophobic minerals. It is more selective than starch.
For adsorption of dextrin (or any polysaccharide) on talc, the most likely mechanism of binding would be through the hydrophobic interaction, involving the hydrocarbon under section of the glucose rings and the hydrophobic face of talc [54].
Carboxymethyl starch is a modified starch etherified with carboxymethyl. Hydroxyl group in the modified carboxymethyl starch forms the chelate with metal cations on the talc surface. The other end of the chelate has a large number of hydroxyl groups that are hydrophilic, thus making talc hydrophilic. At the same time, a large number of hydroxyl groups in carboxymethyl starch molecules can be adsorbed on the talc surface through hydrogen bonding. It enhances the hydrophilicity of talc and decreases its floatability [55].
3.7 Combined depressors
Combined flotation agents are generally more effective than single agent, mainly due to the synergistic effects between agent molecules in the flotation process[92]. According to the literature reports, their main mechanisms of action are composed of the combination mechanism, complexation mechanism, chelation mechanism, coadsorption mechanism, etc. [36]. Several combined depressors are described below.
3.7.1 Combined use of AlCl3 and carboxymethyl starch
Firstly, AlCl3 is added to the ore pulp (with pH 5-6). Then talc surface is electronegative, and aluminum ions and aluminum hydroxide ions are electrostatically adsorbed on the talc surface. The hydroxyl groups in carboxymethyl starch form chelate with aluminum ions and aluminum hydroxide ions adsorbed on the talc surface. The other end of the chelate has a large number of hydroxyl groups that are hydrophilic, which makes talc hydrophilic [55]. AlCl3 and carboxymethyl starch are used as depressors for talc, with low dosage and strong depression effect, while hardly affect the flotation of copper sulfide minerals.
3.7.2 Combined use of ZnSO4 and Na2CO3
The effective depressing components of the combined depressor of ZnSO4 and Na2CO3 for talc were solid state ZnCO3 and Zn(OH)2, and ZnCO3 played a major role. When ZnSO4 and Na2CO3 are used as the combined depressor for talc, the addition of Na2CO3 should be strictly controlled at a level suitable for the formation of solid state ZnCO3, and the initial concentration of ZnSO4 should be higher than 2.5×10-4 mol/L [56].
3.7.3 Combined use of CaCl2 and sodium lignosulfonates
The combined action of CaCl2 and sodium lignosulfonate effectively reduces the content of talc in the chalcopyrite concentrate. The zeta potential measurements, contact angle measurements, and adsorption studies showed that Ca2+ increases the adsorption density of sodium lignosulfonate on the talc surface, with the reduction of the surface potential of talc, and enhancement of the hydrophilicity of talc particles. Thus, it depresses the floatability of talc. These findings were confirmed thourgh XPS analysis. Furthermore, the infrared spectroscopy analysis showed that CaCl2 and sodium lignosulfonate can be chemically adsorbed on the talc surface in strongly alkaline conditions for selective depression of talc [51].
In addition to the cases mentioned above, there are also many other combined agents such as CMC and sodium hexametaphosphate, and CMC and guar gum. A study indicated that the depression effect of CMC on talc can be facilitated in the presence of Ca2+ and CO32+ [93]. Sun [36] developed a combined depressor SY, which is to form a film of poorly soluble compound adsorbed on the talc surface. The combined agents are used to reduce the cost of agents and maximize depression effect, thus maximizing the economic benefits. The main purpose of using combined agents is to reduce the cost of agents and maximize the depression effect of depressors, so as to achieve the maximization of economic benefits.
4. Conclusions
Preferential talc flotation followed by chalcopyrite flotation, depression of talc with flotation of chalcopyrite, bulk flotation of talc and chalcopyrite are the main processes for separation of chalcopyrite and talc. To maximize the recovery of chalcopyrite, depression of talc with flotation of chalcopyrite is the most economical and efficient, which has been applied to the copper ore in Dongguashan, the zinctin- copper polymetallic ore in Dulong, China, etc.
Depressors for talc are divided into organic compounds and inorganic salts. Carboxymethyl cellulose is one of the most widely used organic depressors, and its adsorption mechanism includes electrostatic force, hydrogen bonding and chemical bonding. Chitosan depresses talc by physical adsorption and hydrogen bond adsorption. It is similar for the mechanism of depressing talc by natural gums such as locust bean gum and arabic gum, mainly controlled by hydrogen bond. The adsorption mechanism of lignosulfonate includes hydrogen bonding, electrostatic interaction, chemical bonding and the complexation of calcium ions on the mineral surface. Dextrin produced by starch hydrolysis is easy to interact with talc. The modified carboxymethyl starch forms chelate and hydrogen bonds with talc to depress the talc flotation. Some metal ions can be adsorbed on the talc surface in the form of hydroxyl complexation ions to form hydrogen bonds.
Combined depressors suggest novel synergistic depression functions, which is more efficient than single agent.
In the future, the separation of chalcopyrite and talc will focus on new depressors and the modification of agents. Meanwhile, the development of new depressors for talc needs to consider the properties of associated minerals with talc and copper-bearing minerals. The combined depressors should be developed to maximize the recovery of valuable metals.
Competing Interests
The authors declare that they have no competing interests.