1. Introduction
Over 99 % by weight of the Earth’s crust consists of just ten elements (O, Si, Fe, Al, Ca, K, Na, Mg, Ti, H). Among them, there is iron (Fe), which has been extracted and used by far the greatest amounts of all the metals per year these days. Nevertheless, the quite many minor or trace elements (Si, Mn, P, Al, S, As, Cu, Zn, K, Na, Ti, Cr, V, W etc.) most often mentioned by the ferrous metallurgists, are rarely considered as beneficially extractable ”by-products in the ferrous metals industry”.
During steelmaking, the harmful elements, either metals or nonmetals, must be and are normally really well removed from the steels and transferred into the slags and dust and flue gases [1,2]. Some less reactive metals, also of higher market value as that of copper and tin, are accumulating somewhat in many conventional grades of steels produced in the highest quantities in the world. Their recovery from steels, however, is still an unsolved problem for the extractive metallurgists [3].
Another minor/trace metal is worth emphasizing here is zinc, especially when zinc containing (e.g. hot dip galvanized) steel scraps are processed in electric arc furnaces (EAF steel making). During such type of the secondary steelmaking operations, the highly volatile zinc will accumulate in the dust formed during the meltdown of scrap. Consequently, there is a better chance for the extraction of zinc from the EAF dust, which operation can, in principle, be done easier via hydrometallurgical means, i.e. starting with selective leaching of the EAF dust collected as a ’by-product’ of steelmaking. And, indeed, such an aqueous recovery type approach for the extraction of zinc from such by-products and other zinc containing wastes was also supported by Jha et al. [4] in their quite exhaustive recent review.
In line with that concept, in our present paper we highlight a few additional aspects of dealing with the iron and steel making slags and dust, then we shall summarize the results of our own EAF dust leaching laboratory experiments.
2. Materials And Recovery Methods
2.1 Iron and steelmaking slags and dust
In addition to the dust generated and collected during the iron making and steelmaking, the different kinds of slags are of the greatest amounts of the ’by-products’ in ferrous metallurgy. They are relatively inert, and nowadays almost 100 % of the blast furnace (BF) slags are utilized in many areas, such as cement production, road construction, civil engineering work, fertilizer production, etc. Since the BF slag’s iron content is low, its recovery from it is not important [6,7].
Steel slags, however, are of greater varieties, but the cases of alloy steels (e.g. stainless steels) making slags and ferroalloy slags will not be discussed here, because a great portion of the valuable alloying elements (like Ni and Cr) of such alloy steels are being recycled effectively into the liquid steels both during the primary and secondary (scrap based) high-tech steelmaking technologies of today
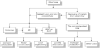
The applications of steel slags (Figure 1) are also many. Table 1 presents an example of the elementary composition of a given steel slag while Table 2 gives another example of the composition of a similar steel slag.
2.2 Recovering manganese from steelmaking slags [8]
As it is the case with almost any aqueous processing techniques to be applied to treat solid materials, first the steel slags as well must be properly conditioned (ground, heat treated or even partially reduced etc.) in order to liberate and make amenable the mineralogical phases to the chemical reagents (aqueous lixiviants). Then, if chosen and applied properly, they can dissolve selectively the designated components. In this case, McIntosh and Baglin [8] chose ammonium carbamate to leach manganous oxide selectively from the pre-treated silicon steel BOF slag according to the flowsheet shown in Figure 2.
Though they were able to prove experimentally that manganese could be recovered from such type of steel slags with ammonium carbamate leaching up to 80 wt.% of Mn and 50 wt.% of Fe, but all in all, the process so developed was not profitable at that time and is so still today.
2.3 Recovering zinc from EAF steelmaking dust
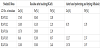
In principle, at least, a similar approach to that of McIntosh and Baglin [8] tested for a given steel slag containing manganese should be more promising to treat zinc containing dust with extremely fine particle size as received from the EAF dust separating unit of the EAF steel mills. However, the electric arc furnace dust with a total Zn content of maximum 35 wt.%, generally also contain many other metals as well, in the form of different oxides, ferrites, and silicates. Further constituents are alkali- and alkaline earth metal compounds as well as minor oxides of further heavy metals and silica. An example of the composition of several EAF dust samples is given in Table 3. These samples were collected during a Joint European Research Project ’REDILP’ [9] from different secondary steel mills in Germany and Hungary. The EAF samples No.: R1…8 and Th1…10 were collected in Germany while the ones denoted by Hu1 and Hu2 were obtained from Hungary. Their mineralogical phases were determined by X-ray diffraction (XRD) analysis and the abbreviations in Table 3 refer to the following minerals:
Ph1: franklinite [(Zn,Fe,Mn)(Fe,Mn)2O4]; Ph2: zincite ZnO; Ph3: magnetite Fe3O4; Ph4: merwinite Ca3MgSi2O8; Ph5: sylvite KCl; Ph6: halite NaCl; Ph7: hematite Fe2O3; Ph8: lime CaO; Ph9: galenite PbS; Ph10: brookite TiO2; Ph11: cristoballite +quartz SiO2; Ph12: calcite+vaterite CaCO3; Ph13: carobbite KF; Ph14: wüstite FeO; Ph 15: witherite BaCO3.
Some of the samples also contained 1 to max 10.5 % (wt.) of the following mineral phases: Ph16: pyrolusite MnO2; Ph17: Pb-ferrite PbFe2O4; Ph18: iron Fe; Ph19: lepidocrocite γ-FeO(OH); Ph20: mayenite 12CaO∙7Al2O3; Ph21: anhydrite CaSO4; Ph22: periclase MgO; Ph23: enstatite Mg2Si2O6; Ph24: dolomite CaMg(CO3)2; Ph25: barite BaSO4; Ph26: thermonatrite Na2CO3∙(H2O); Ph27: microcline KAlSi3O8; Ph28: spinel MgAl2O4.
All in all, the quite many different EAF samples collected and tested by us during the REDILP project contained mainly franklinite [(Zn,Fe,Mn)(Fe,Mn)2O4] between 42 to 57 wt.%, zincite /ZnO/ between 2 to 29 wt.% as well as chlorides and fluorides of sodium and potassium between 3 to 9 wt.% in total as halite, sylvite, and carobbite, among many other minor ingredients.
Generally speaking, any prospective hydrometallurgical approach to treating/dissolve such type of zinc-bearing ’secondary raw material’ should begin with the selection of a cheap and selective enough leaching agent. And, in this metallurgical system, the total number and variety of such chemicals are quite many: there are different acids (H2SO4, HCl, acetic acid etc.), several bases (NH4OH, , NaOH etc.) as well as many other aqueous lixiviants (NH3+(NH4)2CO3, NH3+NH4Cl etc.), which all have already been tested to leach EAF dust samples [10,11]. Recently, Deng et al. [12] even chose and tested trichloroacetic acid (CCl3COOH) for their dissolution experiments and also reviewed a few more other lixiviants.
In the REDILP project, our choice was the ammoniacal ammonium carbonate (AAC), which proved to be effective to dissolve the oxide of zinc, but not its ferrites. Then, the puzzling query has arisen again about how one could increase the yield of zinc without the total chemical decomposition and full dissolution of the zinc ferrites and other contaminants of such rather complex Zn-bearing materials [10]. The additional use of different wet mechanochemical grinding while the AAC is leaching the EAF dust samples also did not increase the yield as much as it was expected before, especially given the development of a new process in itself to produce a marketable zinc compound or pure metallic zinc [9]. Moreover, during our ammonia - ammonium carbonate leaching experiments during the REDILP project, soon it has become quite evident that the AAC leaching residues as well must be further treated somehow, at least to decrease their lead content, which was often as high as around 2 wt.%. Towards that aim, the high-temperature aqueous NaOH digestion approach was chosen based on some relevant earlier research results obtained independently in some other research laboratories [13-19].
2.4 Secondary leaching on the AAC leached residues of EAF dust
The AAC leaching residues collected during the REDILP project and sent to Miskolc for further ’de-leading’ NaOH leaching trials contained (dried samples) about 11 ±2 wt.% Zn and about 2 ±0.2 wt.% lead (dried samples) in the form of different oxides, etc. solid phases. To investigate the recoveries, first of all, lead and zinc from the given residues using high-temperature secondary NaOH leaching, several sets of laboratory experiment were designed and performed by our departments of the University of Miskolc. In compliance also with the reviewed relevant literature data plus having evaluated our preliminary leaching and precipitation trials [20], the following experimental parameters were chosen.
-
Leaching reactor: closed laboratory autoclave of a total volume
capacity of ~0.3 dm3
Autoclave stirring: slow rate mechanical stirring
Autoclave heating: electrical heating jacket set to the given temperature
Concentration of the NaOH leaching liquor: 8 mol•dm-3 (i.e. 8 M)
Solid/liquid ratio: Max. 30 g dried AAC leached residue to 150 cm3 NaOH solution
Minimum residence times: 30 min at any set temperature
Solid-liquid separation: by centrifuging after leaching
Chemical analysis: standard procedure by atomic absorption spectroscopy
The laboratory experiments were performed up to 250°C with several batches of the residues, otherwise having a similar chemical composition (Table 4). These EAF dust samples were obtained from the Bous steel mill then were AAC leached by the IGAS Company, Germany. Each experiment was commenced with a ’fresh’ sample taken from the given batch and digested at a set constant (maximum) temperature.
3. Results And Discussion
The secondary high-temperature alkaline autoclave leaching trials were done in Miskolc, Hungary and a few representative recovery values for lead and zinc are shown in Figures 3 and 4, respectively. The ACC residues were digested with NaOH liquors in a laboratory autoclave and time of residence of each experiment was 30 min.
The effect of temperature (Figure 3) was found to be quite intense up to about 150°C, but the higher temperatures also tested did not alter the recovery rates of zinc considerably. However, by working at still higher temperatures, the recoveries of lead could be increased up to about 80 wt.%. The possible effect of residence times was also checked and about 30 minutes was found to be more than enough in the given laboratory circumstances tested. In these experiments the AAC leached residues were digested in an autoclave at 150°C using 150 ml of 8 mol/L NaOH solutions and the recovery rates of lead and zinc, respectively, are shown in Figure 4.
In addition to the above observations working with hot alkaline liquors of NaOH, one high-temperature digestion trial was also performed with adding iron(III) chloride hexahydrate (No. of Exp.: 13) instead of NaOH following the recommendations of Leclerc et al.[21], just for comparison. Including this Exp.No.13 and a few more alkaline leaching experiments (Exp. No.8-12,14,15) done with some similar residues obtained from other sources are all summarized in Figure 5. In this course of the additional experimental testing, some small amounts of plasma synthesized zinc ferrite were also digested with NaOH solutions of 8 M at 150°C (Exp.No.11,12). This zinc ferrite was produced from iron and steelmaking dust and sludges [22,23], which also contained some lead contaminant (~0.2 %), hence, the lead could be extracted and detected in the NaOH leached zinc ferrite samples as well. The plasma synthesized zinc ferrite had extremely fine (<0.5 μm) particle size, and it must have contained non-stoichiometric zinc ferrites (like Zn0.7Fe2.3O4, Zn0.4Fe2.6O4), magnetite, wüstite and even ZnO particles [22,23].
In one of the last experimental leaching trials, the once NaOH leached residue obtained from Exp.No.3 /RP-150/30/ was then tertiary digested with fresh NaOH solution of 8 M and with similar temperature and residence time (Exp. No.9). It is observed that almost no more zinc could be mobilized, but the lead started to dissolve a bit further.
A kind of diagrammatic summary of the high-temperature autoclave leaching trials on the collected AAC leached residues of EAF dust samples (Exp.No. 1-10,14,15) are shown in Figure 5 where RP refers to the pulverized mixture batch of the three original residues of RP14V 10.1 plus 10.2 and 10.4 (Table 4).
4. Conclusions
After a thorough literature search and our experimental observations, it can be confidently stated, that the extraction of the valuable transition metals, first of all Zn, from the by-products of iron and steel making has been a rather challenging task. While focusing primarily to the EAF dust (mixture mostly of fine size and complex metal oxides) originating from secondary steelmaking, recently even a major European Project ’REDILP’ was accomplished to elaborate an efficient EAF dust recycling technology. In this project the University of Miskolc was an important university participant along with the Otto von Guericke University of Magdeburg together with several other project participants mostly from Germany. According the REDILP Partners’ approach, a mild and relatively selective leaching agent /AAC/ was chosen and successfully applied to solubilize and extract the water soluble portion of zinc in the form of zinc ammonium complexes, however, the leaching residues (with compositions shown in Table 4) still contained high amounts of the undissolved Zn and Pb. Then, to chemically modify and solubilize the zinc containing residues, several high-temperature (hydrothermal) NaOH leaching tests were also carried out in stirred autoclaves with varying leaching parameters. The recovery rates for Zn and Pb showed that relatively short residence times and moderately high temperatures were enough to mobilize (solubilize) about half of the overall zinc content and 75 wt.% of lead from the solid residues. Further dissolution of the rest of zinc with NaOH solutions, however, seemed to be hindered, most probably due to the high-stability structure of those phases in which the remaining zinc is locked in. These phases are identified by XRD measurements as iron zinc oxides (Zn0.945Fe1.78O3.71). Nonetheless, the advantageous de-leading effect of the hot NaOH solutions was experimentally proved, and it can be further enhanced by optimizing the experimental conditions. Moreover, it was also experimentally verified, that combining secondary leaching with the primary AAC leaching or leach-grinding approach will lead to a considerable increase in the overall recovery rates of zinc as well, which metal is the most valuable constituent of the EAF dust.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
Special thanks to the departmental colleagues F. Ferenczi and K. Tisza (Institute of Metallurgy) for performing the high-temperature leaching tests with aqueous solutions of sodium hydroxide. Part of this work had also been financially supported by the European Union in the framework of the CRAFT Project COOP-CT-2004-508714.