1. Introduction
So far, the major effects of environmental pollution by toxic and greenhouse gases such as CO, NOx or combination of NO and NO2, SOx or SO2 and CH4 from industrial processes have been increasingly evident and problematic. As a result, scientists have been interested in oxide based gas sensors recently. At present, there are the two categories of gas sensors: equilibrium and mixed potential gas sensors, semiconductor bulk and boundary gas sensors. In this review, we focus on the latter [1-8]. In the specific meaning, we need to work on bulk conductivity sensors and boundary conductivity sensors, which use iron (Fe) oxide particles with various micro/nanoscale structures, e.g. Fe oxide nanoparticles or microparticles. In one gas sensor device, sensitivity is an important parameter of the sensor in the detection of the concentration of a gas or a mixture of various gases. Here, sensitivity is defined in terms of resistance, conductance, or conductivity of various oxide sensors. It is well known that sensitivity S is defined as [5].
Consequently, the above equation is usefully applied for n-type or p-type metal oxides in the presence of reducing or oxidizing gasses. In addition, R0 and Rgs are the stable values of the resistance of the material before and after exposure to gas. Here, the sensitivity S is defined as [5].
Thus, p-type or n-type semiconductor-based gas sensors sensors are the electrical devices for measuring target gasses or gaseous molecules [6-8]. After chemisorption, reducing gasses inject electrons into an n-type material, thereby creating a negatively charged space charge layer or accumulation region near the surface of the material [1-8]. This has led to increase the conductance of n-type material. For p-type material, the reducing gases extract holes from the oxide material, and hence the conductance is reduced [1-8]. Typically, various materials have been reported for use in semiconductor gas sensors, such as SnO2 (n-type) [9], TiO2 (n-type) [10], ZnO (n-type) [11], In2O3 (n-type) [12], WO3 (n-type) [13-15], α-Fe2O3 (n-type) [16], α-Fe2O3 (n-type) [17], carbon nanotube (p-type) [18,19], Co3O4 (p-type) [20], V2O5 (p-type) [21], CuO (p-type) [22], and etc. Among them, α-Fe2O3 has very large potential for practical use in gas sensors due to its high sensitivity, high stability and sensitivity to various gases, especially toxic gases [23,24,70]. A large number of n- and p-type nano-oxides have been widely investigated for their gas-sensing properties. Among them, Sn and Fe oxides have proven superior for practical application as gas sensing materials films [9,16,17,44]. Additionally, there are various perovskites with the general formula ABO3, typically as SrTiO3 and SrTi(1-x)FexO3 that are promising candidates for high-temperature sensors to detect NO2, CO, and CH4, respectively [6-8]. However, gas sensors must satisfy the strict requirements and standards of sensitivity, selectivity, and stability [1-9]. In addition, they need to show the desirable criteria of short response time, easy processing, long lifetime, and less low-cost technology. Here, metal-oxide gas sensors measure electrical conductivity for the detection of the presence of a target gas. Thus, the change in resistance is proportional to the certain concentration of the target gas in the surrounding air, which allows the determination of the concentration of the target gas. There are various important factors that influence on sensitivity of gas sensors. It concludes particle size and porosity of gas-sensing materials, operating temperature of gas sensors, thickness of oxide film layer, doping effect, and etc. Researchers suggested that sensitivity of gas sensors increased with decreasing particle size, especially with particle size equal to 2LD in the range of 10 nm (LD=Debye length). However, high porosity of gas-sensing oxide materials also leads high sensitivity of gas sensors [1-9], and for environmental trace analysis.
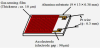
The electric circuit of the gas-sensing measurement is shown in Figure 1 with the special meaningful use of novel Fe oxides. The electric resistance of sensor device [R] is calculated from voltages [V] in raw experimental data [81], and according to the following equation
R = Rgs(VS/V-1)
Here, VS is the supplied voltage of gas sensor. Then, we can calculate the sensor’s response (Rgs/Rair), and concentration of target gases that can be detected in environment. Therefore, sensitivity can be expressed as [1-8].
S=|Rair-Rgs|/Rair=ΔR/Rair or S=(ΔR/Rair)×100%
Correspondingly, ΔR is the change in resistance in the presence of target gas to clean air. To enhance sensitivity, researchers employ metal nanoparticles with desirable size and shape from nm to μm size ranges on the prepared oxides. The key structural parameters of the gas-sensing particles that are of interest to scientists include sensitivity, durability, and stability. Traditionally, platinum (Pt) based nanoparticles are used for enhancing sensitivity of gas sensors but high cost. Therefore, new Fe-based oxide nanoparticles have been quickly developed for gas sensors with low cost. In this context, the Fe oxide-based gas sensors can meet the strict demands of high sensitivity, high selectivity and stability of modern sensor technology with low cost [1-8]. In addition, Fe metal and oxide-based sensors proved short response time, easy processing, long lifetime, and low cost on the market. In recent years, Fe- and Pt-based metal and oxide nanoparticles have played very important roles in many technologies. In energy technology, Pt-based catalysts containing Pt group metalsbased (PGMs) nanoparticles include Ru, Rh, Pd, Os, Ir, and Pt, which are very crucial to develop high-performance catalysts for fuel cells (FCs). For a case of Pt nanoparticles, new Pt nanostructures need to be precisely controlled with large specific surface areas, which may lead to be very appropriate for the commercialization of FCs with new Pt-based catalysts. At present, various oxide materials are potentially developed for various gas sensors to detect hazardous substances. They can be oxide-based sensors or other composite/ multi-component oxide sensors. In particular, Fe oxide with various crystal structures with high durability, stability and sensitivity provide potential opportunities and applications for gas sensors because we can synthesize Fe oxide nanoparticles for large-scale commercialization. In nanoparticle technology, controlling both size and shape of nanoparticles or controlling nanostructures are important to improve their applied properties. For gas-sensor material technology, at this present period, researchers are trying to make the various products with cheap metal and oxide particles for large-scale production of gas sensors with the utilization of noble metals nanoparticles, such as Au, Ag, Pt, and Pd (a small amount) and oxide nanoparticles (a large amount). The role of very tiny noble metal nanoparticles to gas sensing was exactly confirmed [25], such as facile synthesis of Pd nanoparticles [26,27]. In addition, we strongly suggested that the various Fe-based oxide sensors will be developed for sensing applications to detect various gases, typically such as CO, CO2, CH4, O2, H2, and ethanol (C2H5OH) or their mixture [1-8,81,82]. According to the key issues of durability and stability of gas-sensor devices, it is suggested that further investigation of the effects of strain, plastic and elastic surface, surface deformation effect, effects of crystallographic planes of Fe- and Pt-based metals and oxides nanoparticles are very crucial to enhance the catalytic activity and gas sensitivity of the gassensing layer films.
In an extensive short survey, researchers have presented the ability of using Fe2O3 oxide materials in both microstructures and nanostructures for new gas sensors [1]. At present, the technical challenges in controlling micro- and nanoscale Fe metal and oxide systems are the uniform characterizations of size, shape, structure, and composition. To improve selectivity and sensitivity of gas sensors for the detection of CO, CO2, CH4, O2, H2 and C2H5OH in potential gas-sensing applications, new polyhedral Fe2O3 oxide particles with grain and grain boundary structures are proposed for the gas-sensing material layers of gas sensors. In addition, large polyhedral Fe2O3 oxide microparticles can be potentially offered for various sensor devices with the addition of very tiny noble metal nanoparticles for enhancement of both sensitivity and selection to gaseous species. More importantly, they are being promising candidates because of very low cost but very high durability and stability. In the intrinsic range, this α-Fe2O3 nanoparticles material heated in the temperature range of 450 to 1000°C can detect CO, CO2, CH4, O2, and H2, respectively. In general, all the sensor devices with the use of Fe2O3 based materials are suitably compatible to hybrid microcircuit technology through standard semiconductor technology, such as Si and SiC. Thus, the ultimate goals of shaping Fe-based and Pt-based nanoparticles in both size and shape allow us to find out cheap, new, and efficient materials in gas sensing (new sensors), catalysis, energy conversion and storage as well as practical applications in biomedicine (traditional medicine) and nanomedicine (nanotechnology), and especially reducing very high cost of Pt catalyst in fuel cells as well as new practical application for biomedicine. In this review, we show that scientists will face various major challenges of future sensor technology: (i) To control uniform polyhedral shape of metal, alloy and oxide structure in the nano/micro sized range of 1-10 nm and 1-10 μm, (ii) To distinguish their characterization between a nanosized range of 10 nm, and a micro-sized range of 10 μm, and (iii) To apply the preparation processes of rational micro/nanoscale particle heat treatment for the new improvement of their applied properties. (iv) To make the new kinds of large oxide nanoparticles with grain and grain boundary micro/nanoscale structures, e.g. Fe oxides, and investigate the main effects of the deformed shapes and morphologies for the highest ability of enhancing significantly gas sensing, catalytic sensitivity, and unique structural sensitivity to various gases.
2. Synthesis
At present, the bottom-up or top-down approach can be very potentially successfully used for the synthesis of metal oxides for gas sensor applications, especially for Fe2O3 (hematite), and Fe3O4 (FeO. Fe2O3) (magnetite) matrices nanomaterials. We knew that Fe3O4 oxide is regarded as a combination of FeO and Fe2O3 oxides, which are used for gas sensors and related technology [1-8]. Fe3O4 based nanomaterials were prepared by famous equation according to FeCl2 + 2FeCl3 + 8NaOH → Fe3O4 + 8NaCl + 4H2O according to the molar ratio of Fe(II) and Fe(III) precursors amounts equal to 1:2 [8,70,79,93]. Then, Fe2O3 oxide based nanomaterials were formed from the prepared Fe3O4-based nanomaterials by heat treatment in furnace, and in H2/O2 or air according to the known equation 4Fe3O4 + O2 → 6Fe2O3 [8]. The different synthetic methods are briefly discussed [3,4], and all kinds of Fe2O3 and Fe3O4 oxide nano-, and microstructures were reduced by H2 to form a variety of the Fe metal nano-, and microstructures. It concludes vapor phase growth, vapor-liquid-solid mechanism, vapor solid mechanism, solution phase growth, template free synthesis etc for the controlled and shaped synthesis of micro/nanoscale metal and oxide systems.
2.1 Synthesis of metal nanoparticles
Around the world, so far researchers have synthesized metal nanoparticles by chemical and physical methods, such as Cu, Ag, Au, Pt (Figure 2a) [33-36,81], Pd, Ru, Rh, Ir, Os, Ni, Fe, and Co, respectively. In major strategies, they can be supported on Fe or Sn oxides, e.g. low-cost nanomaterials for improvement of gas sensing according to the formation of metal oxide-based heterojunctions. Shaping size and shape of metal and oxide particles can be carried out by modified polyol method with structure-controlling chemical agents as NaBH4, EG (or various polyols), acids, and bases, typically as sodium hydroxide (NaOH), ammonium hydroxide (NH4OH), e.g. industrial chemicals available or other structure-controlling agents. To make Fe3O4 nanomaterials, a mixture of FeCl2 and FeCl3 in the 1:2 molar ratio, and the use of NaOH has been very efficiently used. They can be facilely synthesized in the certain range of 10 and 30 nm because of the highest quantum size effects to structural sensitivity and catalytic activity. However, controlling both size and shape of materials for gas sensor is still difficult to scientists. The polyhedral and spherical shapes of uniform metal nanosystems are considered for enhancement of catalytic and structural sensitivity in gas sensors. In many recent works, scientists have mainly focused on the controlled synthesis of Pt- and Fe- based nanoparticles for catalysis, energy and environment.
2.2 Synthesis of Fe- and Pt- based nanoparticles
In recent years, Pt catalyst has played a key role in sustainable hydrogen economy but very high cost to gas sensor technology. We tried to combine the advantages of Pt- based nanoparticles with high cost, and Fe-based nanoparticles with low cost, and with high durability and stability for gas-sensor applications (Figure 2b and 2c). In most cases, Fe metal element is cheap, abundant, competitive and available for use. The nanostructures with Pt and Fe can be easily created by polyol methods in large-scale production with NaBH4, KOH, NaOH, NH4OH, and other structure-controlling agents. Here, Pt- and Fe-based metal, bimetal, alloy and oxide particles with nano/ microscale structures will be new nanomaterials (NMs) with catalytic and gas-sensing applications for gas sensors. In near future, researchers will continue to work on the controlled synthesis of various micro/ nanoscale Fe oxides in combination with commercialized carbon nanomaterials [1-8] for the development of next gas sensors in the research direction.
2.3 New development of metal and oxide nanostructure
To study gas-sensing characterization, the structural parameters of size, shape, morphology, face, surface, and composition are very crucial to gas-sensing nanomaterials. In the recent highlights, we have successfully synthesized Fe, Pt, Pd, and Rh nanoparticles in the 10 nm range by polyol method with the small additions of some inorganic salts [28,29,33-37,42]. Thus, the structural transformations, the highest quantum-size effect, and the characterization of Fe-, Pt-, and Pd-based catalysts in the size range of 30 nm are of importance in sensing properties, and catalytic activity in electro-catalysis. In the nanosize range of 10 nm, the pure metal nanoparticles show very large surface area, and high quantum-size effect for electrocatalysis and gas-sensing activity [97]. Accordingly, Fe-based bimetal and oxide nanoparticles in the broad ranges of 5000 and 10,000 nm can be synthesized with various mixture, alloy, and core-shell structures, and with excellent advantages of high durability and stability for gas-sensor nano/microscale materials. In this context, we suggest that researchers will further investigate in the gas-sensing properties of n-type or p-type Fe oxides existed in sensor devices according to the controlled and shaped characterization of shape, size, shape, morphology, and structure, respectively.
2.4 New methods of heat treatment
In such new synthesis methodology with wet chemical methods, the heat treatment methods are of critical importance to produce micro/nanoscale particle products with the best advantages of the structures and the properties for gas-sensing materials [1-8]. In such approach methods, noble metal nanoparticles can be added into oxide microstructures, e.g. oxide microparticles for catalysis. In physical meaning, metal nanoparticles can significantly enhance catalytic activity and sensitivity to various gases through heat treatment. The new atomic surfaces were formed in Fe- based nanoparticles from 10 nm and 20 nm etc. to 10,000 nm in the better advantages of enhancement of catalytic activity, selectivity and sensitivity at their surfaces. The very slow heating steps are very necessary to achieve desirable micro/nanoscale materials. Here, the new surface deformation, structural plastic and elastic deformation of the grains of Fe- or Pt- based nanoparticles can formed in the best advantages that lead to be an improvement of the higher selectivity and stability of gas sensors of catalytic activity and gas-sensing at all the surfaces of oxide nanomaterials (NMs) in Figure 2c in life, industrial and academic applications. Here, the high stability of gas sensor devices can be achieved by heat treatment or sintering. This important process can be widely applied for metal and oxide nanomaterials and nanoparticles [28,29]. At present, researchers have tried to produce specific nanostructures of metal oxides for sensing applications. They can be quantum dots, nanotubes, nanorods, nanowires, nanoporous architectures, nano-multilayers, nanocrystalline thin films illustrated. The importance of oxide nanostructures is to control gas-sensing properties of gas sensors [5]. Typically, the structural features with high surface area, high porosity and effective surface depletion modulation are focused in the kinds of the prepared new gas sensors [30]. In future, we also hope that scientists in the related fields will study and govern the possibilities of n-type and/or p-type nature of Fe oxides existed in the same gas sensor devices via heat treatment through new and porous structures containing the gains and the grain boundaries. This will lead to realize new Fe oxide materials with various micro/nanoscale structures, which will be thoroughly applied to the previously mentioned areas, such as gas sensors, energy conversion, and batteries [70,83], when they are suitably used with good combination with various carbon nanomaterials.
2.5 Recent results and achievements
For economical purposes, the inexpensive metal oxides by chemical and physical methods mainly involves in the practical aspects of gassensor technology for green environment, and for our life, which is considered as one of the target priority areas of sensor technology [1-8]. In response to the new developments, gas sensors are properly used to detect and monitor a wide variety of toxic and/or non-toxic gases and vapors from typical industrial processes including toxic and explosive gases, organic and inorganic vapors, humidity, and odors [1-9]. Nonetheless, gas sensors can detect ppb (parts per billion) concentrations of various toxic gases. Typically, they are widely provided for the detection, qualitative and quantitative analysis of carbon monoxide (CO) in the home. In automobiles, oxygen sensors are highly essential for controlling engine combustion, and hence fuel efficiency. For greenhouse cultivation humidity, O2 and CO are monitored and deciphered on using oxide sensors. In food industry, alcohol sensors can be used for control of fermentation processes. As the wide range of applications continues to increase, highly reliable and precise sensors can monitor processes in real time. It is clear that Fe metal and oxide particles with various engineered micro/nanoscale structures become increasingly important to address the major needs and challenges of our global society of controlling natural environments.
2.6 Chemical and physical methods
To most of researchers, the major aim of gas-sensing nanomaterials research is to find out large-scale synthetic methods for noble metal nanoparticles, Fe oxide nanoparticles as well as Fe- or Pt- based metal, alloy, and oxide particles, e.g. nanoparticles or microparticles following the chemical method, such as polyol reduction. In rapid and efficient manners, scholars’ focus will be on Fe- or Pt-based nanoparticles for practical application in gas sensors for the detection of toxic gases in the environment such as CO, NOx, and SOx. However, the various types of nanoparticles can be also exploited in other applications for energy conversion, high-performance batteries [31,32,70], low temperature fuel cells (FCs), [33-36] contrast agents for magnetic resonance imaging (MRI) [37,38]. The synthesis approach followed polyol chemical method to make Pt- and Fe- based particles with various structures of grains and grain boundaries, which researchers have successfully developed in recent years [39-42]. Through the chemical and physical methods, researchers and scholars have very successfully synthesized noble metal (Au, Ag, Pd, and etc) nanoparticles, large polyhedral α-Fe2O3 oxide nanoparticles as well as new Ptand Fe-based nanoparticles and nanostructures under size and morphology control in the desirable ranges from micro to nano according to the ranges of properties and practical applications. To address these problems, the uniform spherical and polyhedral shape and morphology of the prepared nanoparticles and nanostructures can be controlled for direct use in catalysis, environmental application, and biomedicine. It is certain that a major challenge to scientists in the field is the controlled and shaped synthesis of uniform nanosized Fe-, Pt-, and Pd-based nanoparticles with desirable shapes and morphologies. It is hoped that the feasible solution is adopted for the significant enhancement of gas-sensing property through uniform bimetallic core-shell nanoparticles. The noble metal nanoparticles can be used as additives, e.g. sensitive agents for large α-Fe2O3 particles, e.g. as the efficient support materials for an improvement of higher selectivity and sensitivity of gas sensors.
2.7 Metal and Fe- based oxide structures
In this review, we mainly focus on the controlled synthesis of Fe oxide particles with micro or nanostructures for various gas sensors, especially large polyhedral α-Fe2O3 nanoparticles. Here, research goals are to create new nanoparticles and nanostructures for higher catalytic activity, high gas sensitivity and selectivity, high durability and stability with short-response time. The goals are to produce Feor Pt-based metal, alloy, and oxide nanoparticles with Pt utilization, e.g. high cost, and Fe utilization, e.g. low cost, and the main goals to create the large-scale synthesis of Fe- or Pt-based nanoparticles by a facile polyol method. For Fe- and Pt-based bimetallic nanoparticles with alloy and core-shell forms, the formation of thin Pt- or Pt- Pd shells (several nm) as atomic monolayers at nanoscales are challenges to scholars. Therefore, they can be potentially used for the considerable enhancement of sensitivity, selectivity, and activity to gas sensors. Here, we expect that researchers can apply thoroughly the following strategy for the synthesis of these nanoparticles with our central ideas of improvement and modification or discovery of novel materials for gas sensors. It mainly concludes the systems of ideas to the development of gas sensors and other practical applications in our research proposal in Figure 3. It concludes (i) The idea of size and shape effects: Shape and size control of noble metal (Au, Ag, and Pt etc.) nanoparticles (10 nm) must be done and Fe-based nanoparticles in the whole size ranges from nano to micro, especially from 1000 nm to 10,000 nm. The addition of the small amounts of Pd, Pt, Au, Ag, and etc nanoparticles in the range of 10 nm can lead to improve selectivity and sensitivity of gas sensors. (ii) The idea of Fe utilization with low cost: Shaping Fe-based metal, bimetal, alloy, and oxide nanoparticles with polyhedral and spherical shapes and morphologies must be done in the certain micro- and nano-size ranges by facile modified polyol methods. A great deal of attention is paid to the sharp polyhedral α-Fe2O3 oxide nanoparticles with large sizes and morphologies. (iii) The idea of Pt utilization with high cost: Shaping Pt-based nanoparticles in 10 nm (1-10 nm) or 20 nm (1-20nm) must be done for use in the enhancement of sensitivity of gas sensors with Fe oxide nanoparticles. In this nanosized range, metal nanoparticles possess the largest quantum size effects to their characterization of gas sensing, catalytic activity and selectivity; Ptbased core-shell nanoparticles must be done with the thin Pt, Pd, and Pt-Pd for enhancement of sensitivity and activity. (iv) The idea of noble metal utilization: High distribution of pure noble metal-based nanoparticles of 1-10 nm range must be supported on large Fe2O3 particles of 1-10,000 nm for use as sensor materials (Figures 2b,c and Figures 4, 5, and 6). Mixing pure noble metal-based nanoparticles (10 nm) must be done with cheap metal and oxide nanoparticles with large size ranges (100-1000 nm) (1000-10000 nm), especially large α-Fe2O3 oxide nanoparticles. Large metal and oxide nanoparticles and very tiny Pt nanoparticles lead to enhance sensitivity of gas sensors significantly. (v) The idea of oxide combination: Mixing different oxides with α-Fe2O3 oxides can be used for improve sensitivity, durability and stability of gas sensors. (vi) The idea of metal-doped oxides: Modification of large polyhedral α-Fe2O3 oxide nanoparticles can be done with various compositions or the addition of various metal elements. They may be Al, Bi, Cd, Ce, Cr, Co, Cu, Gd, In, Mn, Mo, Ni, Nb, Ru, Ta, Sn, Ti, W, V, Zn, Zr and etc. Therefore, p-type or n-type nature can be strongly changed by various doping effects. Finally, above new approach methods can lead to discover novel, inexpensive, efficient, and high gas-sensing metal and oxide materials with a diversity of micro/nanoscale structures through heat treatment for new-generation gas sensors.
2.8 New developments and methods of materials heat treatment
Through nanoparticle heat treatment, it is evidenced that specialists can enhance applied properties of Pt- and Fe-based metal, bimetal, alloy, and FexOy (FeO, Fe2O3, Fe3O4) oxide nanoparticles with both the nano-grains and the boundaries [79-83]. The exciting particle and surface deformation methods are used for creating Pt- and Fe-based nanoparticles with many advantages of high durability and stability in catalysis, gas sensing, and biomedicine. Therefore, the novelties of significantly relative improvement and modification from preparation processes can be achieved and focused on various major aspects. It concludes: 1. The new controlled synthesis methods are used for large polyhedral α-Fe2O3 oxide nanoparticles as well as Fe- and Ptbased nanoparticles with metal, bimetal, alloy, and oxide structures synthesized at large scales with polyhedral and spherical shapes by modified polyol methods, especially with additions of NaBH4, NaCl, NaI, KI, NaOH, and NH4OH, and others for controlling gas-sensing materials in gas sensors. More importantly, designed core-shell metal-metal, metal-oxide or oxide-oxide structures with the great advantages can be also prepared for gas sensors; 2. The synthesis of large polyhedral α-Fe2O3 nanoparticles of high durability and stability by polyol method and use of various methods of materials heat treatment, e.g. nanomaterials and micromaterials heat treatments are done to make new gas sensing film structures. The α-Fe2O3 oxides with grain and boundary structure and great durability and stability can be prepared for this goal; and 3. The set-up and characterization of a working sensor device can be used for detection various toxic and target gases (Figures 1 and 7), such as H2, CO, C2H5OH, and other typical gases in industrial production and process.
3. New Research Directions of Metal and Oxide for Gas Sensors
3.1 New inexpensive metal and oxide nanoparticles
In the synthesis of inexpensive metal and oxide nanoparticles, many scholars have very successfully controlled their structures. In their characterization and importance, expensive noble metal nanoparticles are Pt, Pd, Rh, Ru, Au, and Ag can be facilely synthesized in the range of 10 nm and 30 nm. Inexpensive metal nanoparticles are Cu, Fe, Co, and Ni metals that can be facilely synthesized in the sized ranges of less than 100 nm, less than 1000 nm, and more than 1000 nm. We emphasize that the most important two metal nanoparticles are Ptand Fe-based nanoparticles as gas-sensing agents, and they can be well shaped in the well-controlled nano- and micro-size ranges with the high yields [70,81-83].
For large potential applications in gas sensors, most of noble metal nanoparticles can be controlled in size, shape and morphology in the 10 nm nanosized range with polyhedral and spherical shape and morphology for enhancement of catalytic and sensitive activity to detection of toxic gases according to new methods of gas-sensing modification on surfaces of Fe oxides by noble metal nanoparticles. Inexpensive metal and oxide nanoparticles with the strict drying and heat treatment processes can be controlled in the size, the shape and the morphology in the 1-10 μm micro-size range with polyhedral and spherical shape and morphology for new generation sensor micro/ nanoscale materials (Figures 2c and Figure 6).
In most of practical applications in catalysis, biology and medicine, metal nanoparticles have the size range of 10 or 30 nm exhibited the largest quantum effect. Thus, the most typical nanosystem of metal nanoparticles needs to be uniform in size, shape, and structure in the nanosized ranges of 30 nm. For example, Pd nanoparticles on NiO support materials can produce a much larger number of active sites to target gases [25-27]. These metal nanoparticles can increase the efficiency of gas-sensing property. A better selectivity of gas-sensing can be achieved by metal nanoparticles. However, the performance, durability, and sensitivity of gas-sensor device depend on the structure of metal nanoparticles on the gas-sensor film. The strong dependence of gas adsorption on the shape and size of metal nanoparticles also leads to improve gas sensors. Thus, large metal oxide nanoparticles can be used as the specific supports for noble metal nanoparticles.
3.2 New large polyhedral α-Fe2O3 oxide nanoparticles
In some recent interesting works, the very stable α-Fe2O3 oxide or hematite has n-type semiconducting properties (Eg = 2.1 eV) prepared for use in gas sensor devices [16,63]. For potential applications in stable and highly sensitive gas-sensor devices, α-Fe2O3 nanoparticles with high durability and stability can be synthesized in a largescale production (low cost) for gas-sensor materials for detection of hydrogen (H2), CO, toluene (C6H5CH3), propane (C3H8), C2H5OH, and liquid petroleum gas (LPG), acetone, and NH3, respectively. Here, the prepared α-Fe2O3 nanoparticles must be well controlled in the uniform characterization of size, shape, and morphology. When grain size of semiconductor oxide decreases, volume ratio of space charge layer to the grain increases, leading to an increase in the better sensor response [43,44]. The controlling methods of the shape and morphology can lead to improve the facile diffusion of target gases into the gas sensing films [45,46]. Therefore, morphology and size of α-Fe2O3 has been controlled to nanosphere [47], nanorod [48,49], nanotube [50], hollow sphere [51-53], and porous structure [54-56], in order to improve the gas-sensing properties of α-Fe2O3. This approach method is to investigate mechanisms for gas sensing in pure α-Fe2O3 sensors with uniform shape and size of α-Fe2O3 nanoparticles. In general, grain size control in for In2O3 thin films is very important gas sensors, and composition and structure control of Sn1−xCoxO2 nanostructures [57]. Thus, large and polyhedral α-Fe2O3 nanoparticles in the size ranges of 1-5 μm and 1-10 μm can be synthesized by modified polyol methods with NaBH4, NaOH, and NH4OH as structure-controlling agents, e.g. industrial chemicals. In most of cases, nanoparticle heat treatment or sintering can be used as a new method for producing atom, surface, and nanoparticle deformations in order to greatly enhance strength, durability and stability of the prepared iron oxide structures for gassensing characterization. The nanoparticle-powder products can be used as the commercial products from Aldrich or Sima-Aldrich. Selectivity of a gas-sensor device with the use of α-Fe2O3 nanoparticles should be decided in the presence of toxic gases containing various concentrations. There are many important gas-sensing mechanisms in the operation of gas sensors. They involve in gas adsorption in physisorption and chemisorption. In these processes, the most commonly observed chemisorbed species on metal oxide surface operating in air is O−2 and/or O− where O− is more active compared to O−2 [3,4]. Reducing gases interact with metal oxides through either direct chemisorption or a surface state associated with the adsorbed oxygen p- or n-type materials. Gas sensors operating based on chemisorption have nominal operating temperatures more than 300 up to 600 or less than 1000°C, e.g. oxygen sensors [3-5]. Therefore, the further investigations of reduction, oxidation, and dissociation of gases are very crucial to improve gas sensors significantly. Figure 7(i) shows the response transients to various gases of the sensor device calcined at 500 and 900°C. The value of the sensor response to various gases was summarized in Figure 7(ii). It was found that sensor devices using polyhedral α-Fe2O3 were very sensitive to hydrocarbons and C2H5OH as compared with gases to be H2 and CO, respectively. However, as shown in Figure 7, the recovery time for the hydrocarbons and C2H5OH was rather slow due to the slow desorption speed of oxidized intermediates of target gases [81]. Such a slow recovery speed of α-Fe2O3 will be improved by doping foreign element or loading catalysts. As shown in Figure 7(ii), the sensor device calcined at 500°C was most sensitive to the detection of C6H5CH3. However, in the case of the sensor device calcined at 900°C, the highest sensor response was obtained with the detection of C2H5OH. Through the use of α-Fe2O3, this tendency can be explained by the significant difference in the porosity of the two sensor devices. In the works, scientists suggest that α-Fe2O3 micro/nanoscale materials proved the very good advantages for potential applications in new-generation gas sensor devices. Figure 8 shows the good dependence of the sensor response on the H2 concentration in the levels from low to high contents with the use of α-Fe2O3 micro/nanoscale materials [61,82]. The sensor response to hydrogen gas (H2) was increased with an increase in the H2 concentration without saturation at high concentrations of H2. In particular, the sensor device calcined at 500°C becomes more sensitive to a change in the H2 concentration due to the small crystalline size of α-Fe2O3. Here, the gas sensor devices with the use of α-Fe2O3 micro/ nanoscale materials prepared by polyol method, and with drying and heat treatment methods [61,82] were exposed to various reducing gases, and compared with their sensorresponse. Typically, H2, CO, C6H5CH3, C3H8, and C2H5OH are chosen for the most typical gassensing measurements [82].
3.3 New large Fe oxide nanoparticles: a comparison of gas sensing
At present, α-Fe2O3 nanoparticles are the main component with addition of FeO, Fe3O4, β-Fe2O3, γ-Fe2O3, and ε-Fe2O3, respectively [1-8,79-83]. We can essentially focus on development of iron oxide nano-materials with high durability and stability and new structures as potential gas-sensing materials for gas sensors, with the addition of other compositions, typically such as FeO, Fe3O4, α-Fe2O3, β-Fe2O3, γ-Fe2O3, ε-Fe2O3 nanoparticles, e.g. minor component, mixing into α-Fe2O3 nanoparticles, e.g. major composition. They will be exactly controlled in both microscale and nanoscale ranges with the use of new concepts of the nanosize grains as well as plastic and elastic effects for enhancement of the abilities of high catalytic activity, selectivity and sensitivity to various gases. For example, FexOy nanoparticle-based gas-sensor devices to detect target and toxic gases, such as SO2, NOx, CO, O2 and CH4, respectively. In addition, β-Fe2O3 nanoparticles show promising candidate for potential applications in chemical sensors for detection of NH4OH in researchers’s scientific motivation [69]. In the various approach ways, Fe oxide particles with the very wide diversity of nanostructures and microstructures can be synthesized by various chemical and physical methods [58,70]. It is well known that the α-, β-, and γ-Fe2O3 oxides were used for gas sensors for detection of ethanol [3,4,70]. In the research approach methods, large and polyhedral α-Fe2O3 oxide nanoparticles in the size ranges of 1-5 μm, and 1-10 μm can be simply synthesized by modified polyol methods with the additions of a certain amount of NaBH4, NaOH, NH4OH, and other industrial chemicals. Nanoparticle heat treatment or sintering can be used as a new method for producing atom, surface, and nanoparticle deformations to enhance strength, durability and stability of the prepared Fe oxide structures for gas-sensing characterization to detect target gases, such as H2, CO, C6H5CH3, C3H8, and C2H5OH (Figure 7). The most common commercial products can be used as the commercial products in various forms of nano/microscale particle powders from Aldrich or Sima-Aldrich. In addition, α-, β-, and γ-Fe2O3 oxide nanoparticles can be synthesized in the ranges of less than 100 nm and 1000 nm, and in a large amount by this method. As an exciting result, the as-prepared products produced by researches [70,80-83] can be compared with commercial products used in gas sensor devices for the detection of toxic gases, such as NO2, CH4, and CO, respectively. At present, α-Fe2O3 nanoparticles are the main component with addition of FeO, Fe3O4, β-Fe2O3, γ-Fe2O3, and ε-Fe2O3, respectively [1-8,79-83]. We can essentially focus on development of iron oxide nano-materials with high durability and stability and new structures as potential gas-sensing materials for gas sensors, with the addition of other compositions, typically such as FeO, Fe3O4, α-Fe2O3, β-Fe2O3, γ-Fe2O3, ε-Fe2O3 nanoparticles, e.g. minor component, mixing into α-Fe2O3 nanoparticles, e.g. major composition. They will be exactly controlled in both microscale and nanoscale ranges with the use of new concepts of the nanosize grains as well as plastic and elastic effects for enhancement of the abilities of high catalytic activity, selectivity and sensitivity to various gases. For example, FexOy nanoparticle-based gas-sensor devices to detect target and toxic gases, such as SO2, NOx, CO, O3 and CH4, respectively. In addition, β-Fe2O3 nanoparticles show promising candidate for potential applications in chemical sensors for detection of NH4OH in researchers’s scientific motivation [69]. In the various approach ways, Fe oxide particles with the very wide diversity of nanostructures and microstructures can be synthesized by various chemical and physical methods [58,70]. It is well known that the α-, β-, and γ-Fe2O3 oxides were used for gas sensors for detection of ethanol [3,4,70]. In the research approach methods, large and polyhedral α-Fe2O3 oxide nanoparticles in the size ranges of 1-5 μm, and 1-10 μm can be simply synthesized by modified polyol methods with the additions of a certain amount of NaBH4, NaOH, NH4OH, and other industrial chemicals. Nanoparticle heat treatment or sintering can be used as a new method for producing atom, surface, and nanoparticle deformations to enhance strength, durability and stability of the prepared Fe oxide structures for gassensing characterization to detect target gases, such as H2, CO, C6H5CH3, C3H8, and C2H5OH (Figure 7). The most common commercial products can be used as the commercial products in various forms of nano/microscale particle powders from Aldrich or Sima-Aldrich. In addition, α-, β-, and γ-Fe2O3 oxide nanoparticles can be synthesized in the ranges of less than 100 nm and 1000 nm, and in a large amount by this method. As an exciting result, the as-prepared products produced by researches [70,80-83] can be compared with commercial products used in gas sensor devices for the detection of toxic gases, such as NO2, CH4, and CO, respectively.
3.4 New large Fe-based nanoparticles with doping basic metals
At present, we think that scientists can focus on development of base metal-doping α-Fe2O3 oxide materials with high durability and stability for gas sensors with target gases, such as H2, CO, toluene (C6H5CH3), propane (C3H8), and C2H5OH (Figure 7), especially detection of toxic gases, such as CO (toxic gas), H2S (very poisonous gas), NO2 (a major air pollutant from exhaust gases), especially toxic gas CO. In one approach, NiO nanofibers doing with 0.18-13.2 at.% Fe by electrospinning have good responses (Rg- Ra)/Ra to 5 ppm C2H5OH, toluene, benzene, p-xylene, HCHO, CO, H2, and NH3 at 350-500°C [46]. The gas response of Fe-doped NiO nanofibers was enhanced due to electronic sensitization because that is the considerable increase in the chemoresistive variation due to the decrease in hole concentration by doping [46]. In the proposed approach, we need to use α-Fe2O3 doping with other metals for the enhancement of gas-sensing response in gas sensor devices. In particular, we suggest that the difference between structure and morphology of α-Fe2O3 particles with doping elements can lead p-type and n-type characterization for gas sensors [1-4]. (i) Systematic synthesis of α-Fe2O3 and/or Fe3O4 oxide nanoparticles in large-scale production by modified polyol methods with NaBH4, NaOH, and NH4OH for gas sensors for the detection of target gases, as H2, CO, C6H5CH3, C3H8, and C2H5OH, respectively. (ii) Metal-doped Fe oxide-based sensors (Oxide: α-Fe2O3, β-Fe2O3, γ-Fe2O3 and/or Fe3O4), (iii) Doping and modifying by basic metal (Al, Bi, Cd, Ce, Cr, Co, Cu, Gd, In, Mn, Mo, Ni, Nb, Ru, Ta, Sn, W, V, Zr and Zn) for Fe-based oxide sensors for the detection of target gases, as CO, CO2, CH4, C2H5OH, C3H8, H2, H2S, NH3, NO, NO2, O2, O3, SO2, acetone, dimethylamine (DMA), humidity, liquid petroleum gas (LPG), petrol, trimethylamine (TMA), smoke, and many other gases.
3.5 New large α-Fe2O3 oxide particles with addition of noble metal nanoparticles
Through the addition of a small mount of noble metal nanoparticles, the concerning gas-sensing properties of large α-Fe2O3 oxide particles can be significantly improved. Therefore, we can focus on the development of synthetic methods of noble metal nanoparticles (Au, Ag, Pt, Pd, Rh, Ru with the ranges of 10 nm (1-10 nm) and 30 nm (1-30 nm) for these purposes. We can focus on development of large polyhedral α-Fe2O3 particles (or β-Fe2O3 particles, γ-Fe2O3 particles) with the size ranges of 1-5 μm (1000-5000 nm), and 1-10 μm (1000- 10,000 nm) for gas sensors with high selectivity, stability, and sensitivity to various gases. Alternatively, Pd nanocrystals have been decorated on crystalline mesoporous NiO nanosheets for a good improvement of the sensitivity of gas sensors to detect H2 gas [25-27]. In the proposed approach, we suggest that the efficient methods of mixing variousparticles are widely used for large polyhedral α-Fe2O3 (and/or Fe3O4) oxide particles in the microscale range 1-10 μm (1000 nm to 10,000 nm) with the proper addition of a small amount of very tiny noble nanoparticles in a 10 nm range (Au, Ag, Pt, Pd, Rh, and Ru) for the better enhancement of selectivity and sensitivity of gas sensors. Noble metal nanoparticles are very important agents for an enhancement of sensitivity. This will improve our understanding of the important mechanisms of gas-sensing properties of oxide nanomaterials in the films of gas sensors. A comparison need to be carefully made between micro/nanoscale particle products via laboratory and commercial products. Importantly, exciting phenomena of plastic and surface deformation of Fe oxide structures under particle heat treatments or sintering treatments are studied in our research. In the enhancement of sensitivity, stability and durability of gas sensors, we suggest that “Particle heat treatments” are very crucial to all the new and unknown properties and discoveries of engineered particles with the nanosized, microsized, and nanomicro-sized structures and ranges by chemical and physical methods. The new method of nanoparticle heat treatment is proposed for making new structures for their potential applications through atomic plastic and elastic surface deformation or nanoparticle surface deformation by high heat treatment. It is of interest to scientists at present for producing micro-nano structures with new grain and boundary of high durability and stability under heat treatment for improving and enhancing the sensitivity and stability of gas sensors. The TEM and SEM methods are commonly used to study the phase transformations in micro/nanoscale systems, especially for the existence of the micronano textures in every particle, and the most important phase transformations of that as-prepared nanoparticles, which includes the crystalline and solidification.
3.6 New multicomponent oxides: a mixture of α-Fe2O3 and other oxides
At present, binary Fe-based oxides, e.g. a mixture of α-Fe2O3 and other oxides are of interest to scientists and researchers. In this research direction, researchers need to focus on development of large polyhedral α-Fe2O3 nanoparticles in the 1-10 μm range for gas sensors with high selectivity, stability and sensitivity to various gases, such as H2, CO, C6H5CH3, C3H8, and C2H5OH, respectively. We can focus on modification and improvement of large polyhedral α-Fe2O3 nanoparticles (major composition) for gas-sensor materials with a small amount of various oxides, such as ZnO (Wurtzite), SnO2 (Rutile), LaFeO3, SmFeO3, TiO2 (Rutile), WO3 [13-15], Ga2O3, and In2O3 [1-8]. Recently, Fe oxide nanoparticles have shown the good sensitivity to hydrocarbon gases, CO and alcohols. The studies showed that γ-Fe2O3 sensor devices exhibited good sensitivity and selectivity to C2H2, H2, and CO, respectively [1-4]. The Fe oxide-based gas sensor devices with higher sensitivity can be possibly improved by the various doping modes with efficient gas-sensing agents, such as Y2O3, SnO2, ZnO. Among typical oxides for gas sensors, hematite (α-Fe2O3) is one of the most important promising Fe oxides with n-type semiconducting properties (Eg=1.9-2.3 eV) for detection of very toxic CO gas. Today, hematite systems are very proper sensor materials due to its nontoxicity, low cost, and high stability under ambient conditions [60,70]. The role of the (100), (111), (110), and (hkl) facets of α-Fe2O3 was shown in gas-sensing properties [61]. Beside the facet effects, the porosity, grain size, grain and grain boundary structure of α-Fe2O3 need to be further studied. Therefore, in our remarks, large and polyhedral Fe oxide particles, e.g. α-Fe2O3 nanoparticles in the size ranges of 1000-5000 nm, and 1000-10,000 nm may be facilely synthesized by modified polyol methods with the additions of NaBH4, NaOH, NH4OH, and other industrial chemicals corresponding to the suitable drying and heating processes. They are the main compositions of gas sensor devices with a small amount of other oxides. At present, the various metal oxides can be synthesized by chemical methods or bought from commercial products. The oxides such as ZnO, SnO2, LaFeO3, SmFeO3, TiO2, WO3, and Ga2O3 can be synthesized by wet chemical methods or commercial products. The methods of nanoparticle sintering or heat treatment are used for the enhancement of selectivity and sensitivity to various target gases. At present, scientists are of very interest in metal and semiconductor oxides, such as tin dioxide (SnO2), zinc oxide (ZnO), titanium dioxide (TiO2), tungsten (IV) oxide (WO3), copper oxide (CuO), cerium oxide (CeO2). Therefore, binary or multi-component oxide sensors, e.g. a mixture α-Fe2O3 and ZnO, or SnO2, TiO2, WO3, and CuO can be used for modified sensitivity, selectivity, and stability of gas-sensor devices. Most importantly, there are very typical oxides for gas sensors as follows.
- Micro/nanoscale SnO2 materials, e.g. traditional materials for gas sensors have shown electrical property regarded as an oxygen deficient n-type semiconductor (band gap: 3.6 eV). Here, SnO2 based gas-sensor devices can detect various gases, such as NO2, CO, C3H8, H2S, H2, and C2H5OH, respectively [1-8,62,63].
- Micro/nanoscale ZnO materials exhibiting as n-type semiconductor (band gap: 3.3 eV) are potentially used gas sensors for detection of various gases, such as H2, O2, C2H5OH, NH3, and NO2 [1-8,62,63].
- Micro/nanoscale TiO2 materials (band gap: 3.2 eV) have been considered as promising candidate for use in gas sensor to detect gases, such as H2, and NH3 [1-8,62,63].
- Micro/nanoscale tungsten oxide (WO3) expressed as n-type semiconductor has an indirect band gap of 2.6 eV. Here, WO3 oxide-based gas-sensor devices can detect NO2 and H2 gases [1-8,62,63].
- Micro/nanoscale copper oxide (CuO) is a p-type semiconductor (1.2 eV). CuO based gas sensors with the addition of CuO can detect NO2, CO, and NH3. SnO2 (n-type semiconductor) can lead to enhance the higher sensitivity and selectivity to H2S [1-8,62,63].
In the proposed explanation, some researchers proved that the gas-sensing mechanism of CuO-nanowires sensor needed to be intensively studied for an improvement of gas-sensing property of gas sensors [3]. When a reducing gas molecule (H2) adsorbs on the surface of CuO, it donates electrons to p-type semiconducting CuO oxide, changes the surface carrier's density, produces a space charge region (Debye length), and results in increases sensor resistance. The adsorption of hydrogen molecule on the surface of metal oxides is known depending on the operating temperatures. When exposes to hydrogen, the hydrogen molecule can adsorb on the pre-adsorbed oxygen and change the sensor resistance. The adsorption mechanisms of oxygen (in air) and hydrogen on the surface of CuO oxide structures were described as the below following equations.
O2 (gas) ↔ O2
O2 (ads) + e− ↔ O2− (ads)
O2− (ads) + e− ↔ 20−(ads)
O− (ads) + e− ↔ 02−(ads)
H2 (gas) ↔ H2 (ads)
H2 (ads) + O− (ads) ↔ H2O + e−
H2 (ads) + O2− (ads) ↔ H2O + 2e−
e− + ho ↔ Null
They suggested that the adsorption mechanisms of oxygen on the surface of metal oxide occurred at a certain temperature range 100- 400°C with the form of O- or O2- [3]. When the CuO layer is exposed to hydrogen gas, the chemical reactions between H2 molecule and O- or O2- releases free electrons. Such theses free electrons neutralize the hole, decrease the carrier density in the surface charge layer and increase the resistance of CuO oxide. In other example of NiOx doped TiO2 thin films of gas sensors, electrical characterization showed that NiOx content as high as 10% wt. is needed to invert the n-type conductivity of TiO2 into p-type conductivity [64]. Some scholars indicated that nanocrystalline xSnO2(1-x)α-Fe2O3 showed very high ethanol sensitivity 845 for 1000 ppm ethanol when the particle size is 10 nm [65]. Therefore, our approach to binary oxides with the use of α-Fe2O3 and other oxides as additives, e.g. their small amount can lead to enhance sensitivity, stability and selectivity of next-generation gas sensors. In addition, sensor device detects a change in the gas atmosphere due to a change in the electrical resistance of an element. Some scientists showed that polycrystalline thin films exhibited better performance than those of single crystals or large grain films [9,21,66- 72], and they also showed the very important related issues of grain and grain boundary oxide structures [73-78]. The recent results of α-Fe2O3 with grain and boundary structure (Figure 2c) have led to create significant opportunity of improving the performance of gas sensors. In all the practical aspects of significance and innovation, gas sensor technology becomes an increasingly important area all over the world. The new and improved gas sensing materials are very important to successful implementation of gas sensors. Gas sensors have very high potential applications in various areas from environmental control to everyday monitoring of such activities as public safety, engine performance, and medical therapeutics. In fact, compact gas sensors can also be found in various industries, such as chemical and petrochemical industries, food processing, semiconductor manufacturing, agriculture, and fabrication industries. They can be necessary to control and analyze process gases in the motor, ship, industrial plant, its employees, and people living nearby. In particular, gas sensors and their technologies allow the detection of toxic and combustible gases in the atmosphere. Therefore, the uses of these devices can prevent disastrous consequences for people away from poisonous gases or smoke in households, pollutant gases forest fire. Today, scientists are trying to deal with environmental pollution by toxic gases, such as NH3, H2S, CO, NOx, and SOx, respectively. In greenhouse effect, gas sensors are widely used for the detection of CO2 and CH4. Gas sensors can also tested in the detection of various gases of the fuel injection into an automotive combustion engine for the benefit of our society and public health. Therefore, the elaboration of gas sensor technologies promoting the reproducibility of the porous film morphology, and the parameters of sensors based on them, is the task of the greatest importance. In next stage, we suggest that scientists largely expect to develop the following new methods/technologies of gas sensors with novel Fe oxide materials [80-83]. Therefore, the new synthetic methods of Pt- and Fe- based nanoparticles need to be applied for controlling new nanostructures with high stability, durability and stability at low cost for gas sensors, fuel cells, high-performance batteries, and biomedicine. The new and polyhedral α-Fe2O3 oxide nanoparticles as promising candidates are simply synthesized for next gas sensors. Thus, fundamental knowledge of the formation of new grain and boundary structure of Fe oxides via nanoparticle heat treatment or sintering will be gained. Through new micro/nanoscale α-Fe2O3 oxide materials, the technology of gas sensor device with Fe oxides will be tested and produced. We also expect that the utilization of Fe oxides with new micro- and nano-structures for next-generation gas sensors will be very beneficial to detect environmental pollution by toxic gases for public safety. Additionally, novel Fe oxide nanoparticles can be potentially applied for next-generation gas sensors with significant improvement of sensitivity, stability and durability to detect environment risks of toxic gases to animal and human. For researchers, the new mechanisms of Fe oxide-based gas sensor will be undoubtedly usefully gained in further studies. In new Fe oxide based micro/nanoscale materials, it can also lead to better predict an improvement of high-performance energy conversion and storage of battery, potential biomedical applications [79,83] in the efficient treatments of dangerous tumors and cancers as well as potential applications of photocatalyst and chemical sensors [70,79,83-86]. In his main contributions [26-29,33-36, 39-42,78-83,87,88], Dr Nguyen and co-workers have developed the new definitions and concepts of self-attachment, elastic and inelastic self-collision, self-aggregation, and self-assembly phenomena of the nanoparticles according to chemical synthetic processes [78,94], and the very complex issues of atomic arrangements in order or disorder inside metal, bimetal, and alloy nanoparticles with defects, stacking fault, dislocation, twin planes etc at 10 nm by HRTEM/STEM methods [36,42]. In addition, the critical roles of heat treatment to the prepared particle products led to particle deformation, i.e. the levels of the deformation of size, shape, morphology, surface, internal structure, composition, other crystal parameters during sintering and final densification, leading to plastic, elastic, and inelastic deformation on both the surface and inside the solidified particles leading the undiscovered new properties for various practical applications [36]. The high durability, high stability, and better strength of the prepared oxide particles after heat treatment at high temperature significantly enhanced are all the best chemical and physical properties of the particles with both micro- and nanostructures with the very complicate issues of atomic arrangements inside the grains of one particle as well as the grain arrangements of grain and grain boundary textures [78-83,87,88]. These important improvements and modifications are the keys for discovering new functional nanoparticles for life, biomedicine, energy, and environment.
3.7 Magnetic sensors and other practical applications
In addition to the various kinds of the new Fe-based nanomaterials proposed for gas sensors technologies, we have being carried out the research programs of two-phase soft and hard magnetic nanomaterials, with new grain and grain boundary structures (Figure 9), and with significant impact on new magnetic nanomaterials and technologies proposed at Kyoto University [87,88]. We have proposed the modified chemical methods with heat treatment processes in optimization and generalization for synthetic processes at liquid-phase, solid-phase with a strong reducing like the various forms of Ca compounds, and interface or internal chemical reactions for producing hard magnetic materials with rare earth, such as NdFe (typically such as Nd2Fe14B, Nd2Fe17B, and their variable modified oxides and alloys), SmFe (typically such as Sm2Fe14B, Sm2Fe17B, and their variable modified oxides and alloys), SmNdFe, NdFeB, SmNdFeB, and their variable oxides and alloys etc in Figure 11 [89-92]. The new preparation processes are different from physical and chemical metallurgy technologies, and other conventional methods and approaches. Recently, Prof Satoshi Hirosawa, the leading researcher, has carried out the studies of interesting topics of soft and hard magnetic Febased oxides and alloys via heat treatments with various kinds of specific gases, such as N2, H2, Ar/H2 etc at high temperature [90,91], at national Institute for Materials Science (NIMS), Japan. In the wide temperature range from room to about 1000°C, the prepared Fe- based particles with the microscales and good sizes, shapes, and morphologies were proved to be kept in existence of grain and grain boundary structures [78-83,87,88].
These proposals will open new ways of making soft and hard magnetic nanomaterials with grain and grain boundary in both nano and microscale ranges by chemical methods and approaches in Figure 6, 9, 10, 11 and 12. The products of the nano and microparticle powders have been introduced for practical applications for life, such as gas sensor technology in Figure 12 [87,88]. Thus, Fe, FeO, Fe2O3, Fe3O4 oxide particles in Figure 10 in respect with their applications can facilely be prepared according to the following equations from 13 to 22 etc, and via efficient heat treatment and gas(g)-liquid (l)-solid (s) chemical reactions in physical and/or chemical metallurgy [8,70,96].
4FeS2(s) + 11O2(g)→2Fe2O3(s) + 8SO2(s)
4Fe(s) + 3O2(g)→2Fe2O3(s)
3Fe2O3(s) + CO(g)→2Fe3O4(s) + CO2(g)
Fe3O4(s) + CO(g) →3FeO(s) + CO2(g)
FeO(s) + CO(g) → Fe(s) + CO2(g)
4Fe(s) + 3O2(g) → Fe2O3(s)
Fe2O3(s) + 3H2( g) → Fe(s) + 3H2O(g)
Sm2O3(s) + 3Ca(s) + 17Fe(s) → Sm2Fe17(s) + 3CaO(s)
Nd2O3(s) + 3Ca(s) + 14Fe(s) + B(s) → Nd2Fe14B(s) + CaO(s)
Sm2O3(s) + 2H2(s) → 2Sm(s) + 3H2O(s)
(Fe3O4 can be regarded as combination of FeO and Fe2O3)
As we can know, the pure Fe particles can be prepared in H2 gas at 900°C according to Equation 19, which are different from the Fe particles prepared in liquid-phase chemical reactions.
In addition to gas sensor technologies for life with the use of magnetic Fe oxides, the above magnetic nanomaterials can be used in a very wide range for new applications and technologies for power applications, such as motors, generators, transformers, relays, switches, magnetic shielding, audiofrequency, radiofrequency etc, and magnetic recording heads as well as permanent magnets [89]. The novel normal and inverse Fe- based spinel oxide ferrites can be used for gas sensor technologies, microwave devices, and permanent magnets.
4. Conclusion
In this review, we have recognized that novel and important Fe2O3 micro/nanoscale materials powders have become the most promisingly practical next-generation materials for gas sensors according to the latest research results and highlights of Fe oxidebased materials with heat treatments, especially for normal and inverse ferrite systems, such as Fe3O4, CoFe2O4, NiFe2O4, i.e. MFe2O4 [70,80-89] for their important use in gas-sensing layers of gas sensors, especially for the most attractive structures and advantages of metal, alloy, and oxide particles with grain and grain boundary. The use of very tiny nanoparticles, such as Pd, Pt-Pd, and etc for the enhancement of sensing gas properties will be new topics for further studies at present [26,27,94,95,97]. In particular, Fe2O3 materials have a large diversity of specific own nano/microscale structures, which are very proper to improve and enhance significantly the gas-sensing properties of the oxide layer films in next generation gas sensor devices, which will be potentially used for the detection and analysis of toxic and non-toxic gases in various industrial processes and environments, which are very beneficial to our health, life, and society at present. The further investigations of gas-sensing properties and adsorption mechanisms of various gases on the surfaces of the heated micro/nanoscale Fe oxide materials need to be carried out through their dependence on operating temperature of gas sensor devices for achieving high stability and durability. Finally, the new developments of Fe, Fe2O3, Fe3O4, FexOy micro/nanoscale materials with various carbon nanomaterials can be further investigated in various gas sensors. Particularly, soft, hard, and soft/hard magnetic materials including magnetic oxides (soft ferrites), Fe based malleable alloys (FeCoV, FeCoCr, etc), AlNiCo-based magnets, rare-earth magnets (SmCo, NdFeB, NdFeTi, NdFeCo, SmCo(H,C,N) based alloys, NdFeB(H,C,N) based alloys, NdFeB(H,C,N) based alloys etc), NdFe11TiX(X=B,C,N,O,F) based alloys etc [98-100], especially as hard ferrites etc [98,99] have a diversity of practical applications for magnetic sensors.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
Author Contributions
Dr N.V. Long and Prof T. Teranishi substantially contributed to the study conception and design as well as the acquisition and interpretation of the data and drafting the manuscript. All the authors partially contributed in discussion. N.V. Long is thankful to Prof Thomas Nann for his open and positive discussion to this paper.