1. Introduction
The orthorhombic alloys are of great technological interest in high temperature applications since they have a higher strength-to-density ratio and better room-temperature ductility and fracture toughness than conventional TiAl alloys [1]. In particular, Ti2AlNb-based alloys exhibit superior high temperature strength and creep resistance with higher deformability. Generally, Ti2AlNb-based alloys consist of O and B2 (ordered bcc) phase with small amount of α2 phase (ordered hcp). Due to the combination of the O and B2 phase, excellent room and high temperature mechanical properties can be obtained. However, their application in structural components at elevated temperature is still very limited because of unsatisfactory high-temperature oxidation resistance, especially above 700°C [2,3].
The oxidation products of Ti2AlNb-based alloys consist of an alumina-rich outer layer, which is not fully dense due to the presence of TiO2 and AlNbO4 [4]. In addition, oxygen and nitrogen contribute to subsurface brittleness because of interstitial solutes or formation of oxygen- and nitrogen-containing phases [5]. The presence of surface oxides and oxygen-enriched layer degrades mechanical properties. Moreover, bcc phase to O phase transformation results in sharp drop in creep resistance. It is reported that chemical composition has significant effect on the oxidation behavior of Ti2AlNb alloys. It was shown that alloying could effectively improvethe oxidation resistance of Ti3Al and TiAlby addition of Nb, Mo, W, and Si [4]. In particular, the role of volatile MoO3 oxide which can reduce the oxygen solubility is questioned. Also, the effect of W which enhance solid solution hardening is not known yet. In present work, in order to provide a fundamental understanding and experimental base for the development of Ti2AlNb-based alloys with enhanced oxidation resistance, the oxidation behavior of Ti-xAl-yNb-zM (M = minor element such as Mo, W, or Fe) was investigated 600 and 800°C. The formation of oxide layers and the effect of chemical elements on the oxidation behavior were studied. The oxidation kinetics and the corresponding mechanisms were discussed.
2. Materials and Experimental Procedures
2.1 Materials
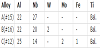
All the alloys studied in present work were based on the ternary composition Ti-22Al-27Nb, which exhibits excellent creep resistance. W, Mo, and Fe were introduced in these alloys, as elements capable of improving high temperature oxidation resistance. Detailed compositions are listed in Table 1. All the alloys were heat-treated at 1150°C for 1hour and furnace cooled to 800°C, and then annealed for 100 hours followed by air cooling. Figure 1 presents the resulting microstructures composed of ordered B2, Ti2AlNb, and α2. Here, B2 phase volume fraction is controlled to equal to each other. Lamellae thickness and morphology are different according to composition.
2.2 Experimental procedures
The samples for thermo-gravimetric test were machined into the rectangular shaped specimens with the dimension of 10 mm length, 5mm width, and 2mm thickness. Surface of the samples were ground with 2000 grit SiC paper and polished. The edge lines were slightly rounded to minimize stress accumulation. The thermogravimetric tests were performed at 600°C and 800°C in dry air using a Setaram machine (Caluiere, France). Heating rate was 50°C/min, and temperature deviation during the testing was below ±1°C. The diffusion profiles of each chemical element were examined by electron probe X-ray micro-analysis (EPMA), JEOL electron microscopy.
3. Results
3.1 Thermo-gravimetric test
Mass gain versus time curves for isothermal oxidation of the three alloys in air at temperature of 600°C and 800°C are shown in Figure 2. Obviously, the oxidation rate of all the specimens increased with the temperature. At 600°C, the kinetics of all materials obeyed a parabolic oxidation rate law for exposure times up to 100 hours. The oxidation kinetics was very fast from the initial starting condition as compared to those observed at higher temperature of 800°C. Sudden changes in slopes, implying surface cracks in the oxide scale, were not observed for all the specimens. Ti-25Al-14Nb-2Mo-1Fe exhibited best oxidation resistance among the three alloys. At 800°C, similar parabolic oxidation rates were foundin Ti-22Al-20Nb-2W and Ti- 25Al-14Nb-2Mo-1Fe. Again, 25Al-14Nb-2Mo-1Fe exhibited best oxidation resistance. In contrast, Ti-22Al-27Nb exhibited break-up behavior and oxidation rate increased dramatically with exposure time. Several transition points are noticed in the mass gain vs. time curves, which indicates rapid oxidation diffusion into the material.
where ΔW is weight gain, A is unit surface area of the specimen, kp is parabolic oxidation rate constant, and t is the oxidation time. The oxidation constants can be obtained by a regression fitting on a weight gain per surface area versus time plot. The oxidation constants of Ti- 22Al-20Nb-2W and Ti-25Al-14Nb-2Mo-1Fe are marked in Figure 3 showing the parabolic rate constants of various Nb containing intermetallic alloys surveyed from literatures. In this plot, the parabolic rate constant of Ti-22Al-27Nb was not indicated because of the non-parabolic behavior at 800°C. It is known that the addition of Nb to orthorhombic alloy improves their oxidation resistance by doping effect of the oxide scale [6]. But, beyond 15% at.% Nb, such a beneficial effect decreases noticeably and the oxide scale appears less adhesive and more porous [4]. Compared with the previous report on Ti-25Al-xNb, the rate constant of Ti-25Al-14Nb-2Mo-1Fe is little bit lower. Therefore, the addition of 2 at.% W, or 1 at.% Mo and 2at.% Fe seems to decrease the parabolic rate to some degree.
3.2 Microstructure observation
Microstructures after isothermal exposure at 800°C for 100 hours were examined. Although all the alloys exhibited thick oxide layers, their thickness and structure were quite different. Outermost oxide layer of Ti-22Al-27Nb was very thick and has many cracks (Figure 4a), while that of Ti-22Al-20Nb-2W was thin and relatively dense (Figure 4b). However, the second layer beneath outermost layer looked very porous and had columnar like structure. Ti-25Al-14Nb-2Mo-1Fe showed thinnest outermost layer and inter-layers were dense although they still contains some cracks (Figure 4c).The chemical composition analysis of the oxidized layer was performed on the cross-sectional observation using SEM with EDS and summarized in Table 2.
Figure 5 shows EPMA investigations of the metal/scale regions were carried out. For all specimens, oxygen and nitrogen were detected in outermost layer. Obviously, the oxygen would be present as TiO2 phase. However, the nature of nitrogen is not still unknown. However, both TiN and NbN were the most possible nitride phase to be formed [7]. In the Ti-22Al-27Nb, layer thickness of TiO2 was very thin. In contrast, below the TiO2 layer, relatively thick Nb and Al area was found. Considering EDS result, the Nb rich layer is assumed to be Al3Nb [8]. It is thought that Nb reduces the amount of Ti and Al in oxidation scale by impeding the diffusion of the elements. As for the Ti-22Al-20Nb-2W, relatively thick Al2O3 layer was observed. Contrary to Ti-22Al-27Nb, Nitride layer was located below the Al2O3 layer. In addition to that, Ti, W, and Nb rich layer was found at similar location. This indicates that lowered Nb content increased Al diffusion so that protective Al2O3 formed on outer most layer. Ti-25Al-14Nb- 2Mo-1Fe demonstrated similar behavior but their Al2O3 layer was much thinner and denser. Noticeable Nb, Ti, Mo rich layer was not observed. Some isolated Fe rich particles were detected around outer surface area but their fraction was not very high. Figure 6 presents schematics explaining the structure of oxide layers.
These results imply that the initially formed Al2O3 layer functions a very important role in the oxidation behavior of the alloys by retarding the inward diffusion rate of oxygen. In contrast, the effect of Mo, Fe, and W on the oxidation rate appears to be not very significant. The difference among the three specimens is mostly attributed to the chemical composition of Nb. Considering the remarkable difference in oxidation rate between Ti-22Al-27Nb and Ti-22Al-20Nb-2W, Al itself seems not to affect much the formation of protective Al2O3 layer.
4. Conclusion
Three different TiAl2Nb alloys were produced. All the alloys obey parabolic oxidation kinetics at 600°C for exposure time up to 100 hrs. Similar behavior was observed at 800°C too. However, However, Ti−22Al−27Nb exhibited abrupt increase in mass gain after 40 hrs.
Ti-25Al-14Nb-2Mo-1Fe demonstrated more excellent oxidation resistance than the other two alloys. Oxide layer was varied with Nb content which modify the formation mechanism of Al2O3 oxide layer by hindering the diffusion of Al. In contrast, the effect of Mo, Fe, and W on the oxidation behavior was not very significant.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
Author Contributions
All the authors substantially contributed to the study conception and design as well as the acquisition and interpretation of the data and drafting the manuscript.