1. Introduction
Lactoferrin (LF) is a multi-function globular protein (80 KDa) present in many biological fluids such as colostrum. Milk, plasma, bile, amniotic fluid, urine, tears and saliva [1-2]; moreover, it represents a peculiar component of secondary granules of neutrophils [3]. By its capability to bind iron, LF exhibits a broad spectrum of biological activities such as antimicrobic [4-6] or anti-mycotic, antiparasitic and anti-viral [7-11]. Together with this well documented iron-chelating activity, LF is able to modulate inflammatory processes by an anti-oxydative action [12-14]; more recently, it has been shown that Lf promotes the bone growth stimulating the proliferation and differentiation of osteoblasts and inhibiting their apoptosis as well as osteoclastogenesis [15-17]. Finally, it has been suggested that LF is able to inhibit cellular proliferation with a suppressive effect on tumours, either in vitro either in vivo [2,11,18-20], although the effective molecular mechanism is still unclear.
In normal human adult and fetal tissues, LF has been immunohistochemically documented in many organs such as kidney, stomach, pancreas, salivary glands, liver, bone marrow and skin [2,21]; moreover, LF immunoreactivity has been also reported in embryo-fetal bone and cartilage tissues, but not in adult ones [22-24]. However, in human neoplastic conditions, LF has been extensively investigated in order to correlate its presence with the pattern of other functional neoplastic markers as well as to determine its possible prognostic role in tumors [25-26]. Therefore, we have thought to be of interest to report herein an immunohistochemical analysis of LF in a cohort of primary and metastatic neoplasms occurred in the bone by using a monoclonal specific antibody.
2. Materials and Methods
LF immunoexpression was investigated in one hundred twelve neoplastic specimens, of which 82 human primary bone and cartilage and 30 bone metastatic lesions obtained through curettage or surgery from an equal number of patients (59 males, 53 females; age range 9-92 years; mean age 41.02 years). The cohort was represent by 10 giant cell tumours (GCT), 7 osteoid osteomas (OO), 6 ossifying fibromas (OF), 34 enchondromas (EC), 6 osteochondromas (OC), 5 chondroblastomas (CBL), 5 chondrosarcomas (CS) (three chondroblastic type and two chondroid type), 3 chondromixoyd fibromas (CMF), 3 osteosarcomas (OS), 1 myeloma and 2 adamantinomas; moreover, bone metastases, surgically taken were obtained from files of our Department, the primary carcinomas of which included breast (10 cases), prostate (6 cases), kidney (4 cases), lung (4 cases), colon (2 cases) and uterus (4 cases). Data concerning the site of occurrence of primary bone tumours as well as the primary corresponding carcinomas producing bone metastases were available.
Samples of unaffected human bone and cartilage tissues were taken at autopsy from 25 fetuses (ranging from 8-34 weeks of gestation), nine of which obtained from fetuses below the 13th week of gestation by legal voluntary termination of pregnancy. Moreover, normal bone and cartilaginous specimens were taken at autopsy from ten adult (mean age 70.4 years) after death from vascular accidents. All these bone fragments were utilized as tissue controls.
The patient’s personal details were non-identifiable and all the patients had provided written consent to their medical information being used for research purposes, according with the Helsinki declaration.
All samples were fixed in 10% neutral formalin for 24h at room temperature (RT) and then embedded in paraffin at 56°C. When neoplastic samples exhibited areas of mineralization, a decalcification procedure using formic acid 5% or EDTA 5%, pH 7.4, for a period not longer than 24 hours was performed. From each tissue block, 4-μm-thick sections were cut and mounted on silane-coated glasses, then dewaxed in xylene and rehydrated in graded ethanols. Parallel sections were stained routinely with haematoxylin/heosin and with Perls’ Prussian blue ferrocyanide. Antigen retrieval, by heating slides placed in 0.01 M citrate buffer Ph 6.0 in a microwave oven for 3 cycles x 5 min, was performed before adding primary antibodies. For the immunohistochemical study, sections were treated in a moist chamber: 1) with 0.1% H2O2 in methanol to block the intrinsic peroxidase activity (30 min at RT); 2) with normal sheep serum to prevent unspecific adherence of serum proteins; 3) with monoclonal primary antibodies against (anti-human) LF (Clone 1A1; Biodesign International, USA; w.d. 1:75; 60 min at RT) and Ki-67 antigen (MIB-1; DakoCytomation; 1:200) 4) with sheep anti-mouse immunoglobulin antiserum (Behring Institute; w.d. 1:25; 30 min at RT); 5) with mouse anti-horseradish peroxidase-antiperoxidase complexes (Dako Cytomation; w.d. 1:25; 30 min at RT). For the demonstration of peroxidase activity the sections were incubated in darkness for 10 min with 3-3’ diaminobenzidine tetra hydrochloride (Sigma Chemical Co., St Louis, MO, USA), in the amount of 100 mg in 200 ml 0,03% hydrogen peroxide in phosphate-buffered saline (PBS). The nuclear counterstaining was performed by Mayer’s haemalum.
Finally, to test the inter-run variability of LF immunostaining, renal tubular structures within normal kidney samples as well as portions of the parotid gland were utilized as positive control in every run. To test the specificity of LF immunostaining in order to deny the possibility of non-specific reaction, serial sections of each affected specimen were tested by replacing the specific antiserum by either PBS, normal rabbit serum or absorbing with excess of purified human LF from human liver and spleen (Sigma Chemical Co.) as well as with pre-absorbed primary antibody: the obtained results were negative.
Immunostained sections were estimated by light microscopy using a x20 and x40 objective lens and x10 eyepiece. Two pathologists using a double-headed microscope performed the assessment of LF immunostained sections on a consensus basis. The percentage of stained neoplastic cells (area of staining positivity: ASP) was graded as follows: 0 (no staining); 1 (>0 to 5%); 2 (>5 to 50%) and 3 (>50%). In addition, the intensity of staining (IS) (weak=1; moderate=2; strong=3) was also taken into consideration. Successively, a LF intensity-distribution (ID) score was calculated for each case by multiplying the values of the ASP and the IS, according to that elsewhere reported in literature [27].
Ki-67 staining score, revealed by MIB-1 antibody, was evaluated by counting the percentage of positive nuclei per 1000 neoplastic cells in up to 10 representative fields of the whole neoplastic portions; all degrees of nuclear staining intensity were taken into consideration. The median labelling index (LI) (Ki-67 LI=2%) was utilized as the cutoff point to determine low and high Ki-67 expression.
Since the measurement of how many people know about different aspects of HIV/AIDS is crucial for designing proper prevention tools, especially for those who may be in contact with infected individuals and patients with AIDS.
The possible statistical correlations between LF immunoexpression and clinico-pathological parameters (age and sex of the patient, site of the tumours) as well as the Ki-67 LI were investigated using Chi-squared test or the Fisher's exact Test, as appropriate. Probability (P) values less than 0.05 were considered to be statistically significant. Data were analysed using the SPSS package version 6.1.3 (SPSS Inc., Chicago, IL, USA).
3. Results
Routinely stained hematoxylin-eosin sections revealed an adequate morphology, allowing to confirm the histologic diagnosis in all examined cases. either in the primary neoplastic lesions as well as in the bone metastatic deposits.
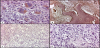
In primary bone tumours, LF immunoreactivity as whole was evident in 21/82 cases (25.60%), independently from Perls’ reactive deposits; in detail, with a variable ID-score, LF immunolocalization was encountered in 10/10 GCT (Figure-1a), 5/7 OO (Figur-1b), 0/6 OF, 0/34 EC, 0/6 OC, 3/5 CBL, 0/5 CS (Figure-1c), 3/3 CMF, 0/3 OS (Fig.1d), 1/1 myeloma and 2/2 adamantinomas (Table-1). Regarding bone metastatic lesions, LF immunostaining was encountered in 14/30 cases (46.6%), mainly due to prostatic, renal (Figure-2a), uterine and colonic carcinomas, while the positivity was reduced in metastases from breast carcinomas (Figure-2b) and it was completely absent in lung cancer (Table 2).
In all cases the LF immunostaining was mainly localized in the cytoplasm of the neoplastic elements and occasionally in the nuclei of the same cells.
With reference to the normal foetal bone-cartilage tissue, LF immunolabelling was found in all foetuses and it was mainly localized in the osteoblasts present in the calcified cartilage area as well as in the nucleus of condrocytes encountered in the cartilage matrix adjacent to the calcified area; a considerable decrease of LF immunostaining was encountered by 24th and up to 29th week of gestation. In addition, no LF expression was in osteocartilagineous tissue after the 30th week and up to 34th week, similarly to that observed in all adult bonecartilage tissue samples.
LF was evident in renal tubular structures, parotid ductular/acinar portions as well as in secondary granules of neutrophils utilized as positive controls.
No statistical correlations between LF immunoreactivity and the other investigated parameters, including age, gender, site of lesions as well as Ki67 LI.
4. Discussion
A variable immunohistochemical expression has been previously elsewhere reported in various human neoplasms, such as carcinomas of the parotid [28], prostate [29], breast [30,31], thyroid [32-34], stomach [35], colon-rectum [36], gallbladder [37], astrocytomas and multiforme glioblastomas [38], skin [39], endometrium [40] and kidney [20].
Recently, it has been documented an improvement of bone health by the use of LF, since this iron-binding protein stimulates the proliferation, differentiation and survival of osteoblast, significantly increasing the mineral apposition and bone formation [16-17,26,41]. In the corresponding pathologic neoplastic bone and cartilage samples, we have already reported some intriguing findings regarding LF immunoreactivity in small cohorts [22-24]. In the present study we have performed a systematic investigation regarding LF immunostaining in primary and metastatic bone tumours. Although LF immunolocalization exhibited a variable intensity-distribution score, CBL, CMF, GCT, OO, myeloma as well as adamantinoma appeared to be reactive, while no staining was detected in EC, OC, OF, CS as well OS. However, the appearance of LF in foetal bone tissue allowed to hypothesize a potential role for LF as oncofetal marker [25]; nevertheless, the quite heterogeneous distribution of LF in bone tumours as well as its independent presence in relation to the biological behaviour seem to be in contrast with the above mentioned role.
Moreover, we have immunohistochemically detected a variable amount of LF in metastatic neoplastic bone lesions of 14/30 cases, mostly attributable to primitive prostatic, renal, uterine and colonic carcinomas, while its positivity appeared to be gradually reduced in secondary deposits from breast carcinomas and, finally, it was completely absent in lung cancer. It should be hypothesized that the negative LF immunoreactivity in small metastatic cohorts was correlated with undifferentiated or less differentiated variants of primary carcinomas [2,20,26,40,42].
The source of LF in human malignant primary and metastatic bone tumours has not been still clarified. It is noteworthy that LF expresses a high affinity for iron, an essential nutrient for cells that are dividing rapidly such as tumour cells, being involved in metabolic processes such as oxydative phosphorylation and RNA and DNA synthesis [43-44]. So, neoplastic elements might produce LF in order to make a greater amount of iron available for their turnover, as we previously suggested [14,18,37-38,45]. Otherwise, the localization of LF in neoplastic cells may not represent the results of an intrinsic cellular synthesis, being instead determined by a defective or functionally impaired LF-receptors, elsewhere demonstrated in human neoplastic cell lines [39-40]. However, some additional functions for LF have been suggested in human carcinogenesis, such as programmed cell death, inhibition of angiogenesis, regulation of cell cycle protein expression and activation of immune cells [2,22-23]. Indeed, LF is able to trigger the apoptotic process by the activation of caspases 3 and 8 as well as the FAS signaling pathway [25,46]. In addition, LF has been considered a protein able to block the endothelial function in vitro and in vivo, probably by the stimulation for IL-18 production [23,47-48]. Finally, it has been suggested that LF promoted growth arrest either at the G1 to S transition in breast cancer cells [47] as well as at the G0-G1 checkpoint in oral and neck cancer cells [48]. Taking into consideration the above mentioned anticancer functions of LF, several studies proposed an exogenous nutritional and therapeutic treatment with LF and its derivatives [49-53]. None of these studies, however, reported a consistent outcome with regard to the mechanisms underlying the anticancer effects of LF. Therefore, we probably need additional analyses concerning new applications of LF in clinical oncology either for its nutraceutical function either for its capability to potentiate chemotherapy.
Competing Interests
The authors have declared that no competing interests exist.
Author Contributions
All the authors substantially contributed to the study conception and design as well as the acquisition and interpretation of the data and drafting the manuscript.