1. Introduction
For superficial esophageal neoplasms (SENs), endoscopic submucosal dissection (ESD) is superior to conventional endoscopic mucosal resection in terms of curative treatment [1-3]. Currently, ESD is indicated for SENs confined to the mucosal layers (pT1a) [4]. Due to a substantial risk of lymph node metastasis (LNM), radical esophagectomy with lymphadenectomy is still regarded as the standard treatment for SENs invading to the submucosa (pT1b) [5]. However, to assess the depth of tumor invasion accurately before the ESD is difficult. Endoscopic ultrasound (EUS) is probably the most frequently used tool but it may not be sufficient to distinguish mucosal from submucosal cancers [6,7]. Therefore, it is inevitable that some patients with cT1aN0M0 SENs undergoing ESD would have pathologically upgraded pT1b cancers after the ESD. On the other hand, patients with cT1bN0M0 SENs may receive ESD rather than surgery because of high surgical mortality and morbidity rates, and poor quality of life after the surgery [8,9]. As a result, some of these patients may have pT1b cancers after the ESD. How to manage these patients with pT1b SENs after the ESD appropriately has not been well elucidated [10-13]. Therefore, we conducted this study to report the outcomes of the patients with pT1b esophageal cancers, with or without adjuvant therapy after the ESD.
2. Patients and Methods
2.1 Study population
A cohort of patients with superficial esophageal squamous cell carcinomas undergoing ESD in Chang Gung Memorial Hospital- Linkou Medical Center was retrospectively identified from a computer database between May 2013 and November 2017. A total of 23 patients with 25 SENs who were diagnosed as pT1b cancers after the ESD were enrolled in this study. In our institution, the major indication for ESD was cT1aN0M0 SENs. However, those patients with cT1bN0M0 SENs who refused esophagectomy or concurrent chemoradiotherapy (CRT) also underwent ESD as a primary treatment. Therefore, the clinical stage was cT1aN0M0 in 17 patients (74%), cT1bN0M0 in 4 patients (17.4%), and cT1N0M0 in 2 patients (8.6%).
2.2 Clinical staging
Preoperative images for clinical staging included EUS, chest computed tomography (CT) scans, and integrated fluorodeoxyglucose positron emission tomography/CT (PET/CT). The invasion depth of the SEN was determined based on the results of EUS: cT1a was the SEN involving the mucosa but not the submucosa; cT1b was the SEN involving the submucosa but not the muscularis propria. Positive nodal staging by EUS was defined as lymph nodes more than 5-mm in the shortest dimension that was spherical and had distinct borders, or by CT was those more than 10 mm in the shortest dimension [14]. The results of the three imaging studies were compared, and any discrepancies were resolved by consensus at a meeting following a further imaging review.
2.3 ESD procedures
All ESD procedures were performed by a single endoscopist (Tsou YK). The details of the ESD procedures were similar to those described in our previous reports [15,16]. The submucosal injection fluid was a glycerol solution plus indigo carmine with or without epinephrine (0.0004%). ESD was performed mainly using an insulated tip 2 knife (KD-611L, Olympus). En bloc resection was defined as completed target resection in one piece.
2.4 Pathological staging
All specimens were pinned on a cork and fixed with 10% formalin. The specimens were embedded in paraffin, cut into parallel 2-mm serial sections, and stained with hematoxylin and eosin. All specimens were examined microscopically for histological type, depth of invasion, lymphovascular (LV) invasion, and resection margins by an experienced pathologist (Chuang WY). The depth of invasion in submucosa was divided into SM1 when the tumor infiltrating the submucosa up to 200 μm; and SM2, when the tumor invading more than 200 μm [4]. Completeness of resection was expressed as R0, when all resection margins were free of tumor cells.
2.5 Adjuvant therapy and Follow-up
Adjuvant therapy included esophagectomy with lymphadenectomy or concurrent CRT. It was our protocol to give the patients adjuvant therapy after the ESD for the following criteria: (1) positive vertical resection margins; (2) presence of LV invasion in the resected specimens; or (3) any cancer with SM2 invasion. To receive surgery or CRT was the patient’s discretion. If the patient refused adjuvant therapy, close observations were offered.
The adjuvant CRT consisted of two cycles of cisplatin 60 mg/m2 on day 1, and continuous infusion of 5-fluorouracil 1,000 mg/m2/day on day 1 to 3, repeated every 4 weeks with concurrent radiotherapy of 45 Gy in more than 20 fractions.
During the follow-up period, esophagogastroduodenoscopy with narrow band images, with or without Lugol’s staining was carried out every 3-6 months and chest CT scans every 6-12 months for all patients. The metachronous neoplasm was defined as a neoplasm that was detected at the esophagus other than the resection site (the scar area). Local recurrence was defined as a neoplasm that was detected at the site of resection and con-firmed histologically. Recurrent tumor included local recurrence, LNM, or distant metastasis. The follow-up data were updated in December 2017.
3. Results
The patients who had more than one SEN were classified according to the SEN with the greatest invasion depth. The clinical features of the patients are listed in Table 1. The mean patient age was 56.3 years; 22 patients (95.7%) were man. Pre-existing major comorbidities included concurrent cancers (n = 10, 43.5%), previous cancers (n = 9, 39.1%), and liver cirrhosis (n = 8, 34.8%). The concurrent or previous cancers were mainly head and neck cancers. All patients had an Eastern Cooperative Oncology Group performance status score of 0 or 1 [17].
The results of the ESD are listed in Table 2. None of the patients experienced serious complications such as major bleeding or perforation which were related to the ESD. The mean resected specimen length was 4.6 cm. Theen block resection rate was 100%. Theen block plus R0 resection rate was 82.6% (19/23) due to four patients with positive vertical resection margins (all were SM2 cancers). As to the invasion depth, four patients (17.4%) had SM1 cancers and 19 patients (82.6%) had SM2 cancers. The mean depth of tumor invasion was 0.79 mm (range, 0.1-1.6 mm). With regard to the histological grading, none had well-differentiated cancers, nine patients (39.1%) had moderately-differentiated cancers, and 14 patients (60.9%) had poorly-differentiated cancers. Four patients (17.4%, all with R0 resection) had LV invasion in the resected specimens.
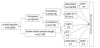
The outcomes of the patients after the ESD were demonstrated in Figure 1. According to the protocol, seven of the eight patients with positive resection margin or LV invasion in the specimens received adjuvant therapy (four for surgery and three for CRT). The other one patient (positive vertical resection margin) refused adjuvant therapy. He was followed-up closely and died of pneumonia 35th month after the ESD. There was no recurrent tumor during the mean follow-up period of 30.1 months in this group of patients. Two of the remaining 15 patients with R0 resection without LV invasion received adjuvant therapies (one for surgery and the other for CRT) according to the protocol. The other 13 patients (56.5%, 11 with SM2 cancers) received close observations only. Two of the patients died of the concurrent head and neck cancers 5th month after ESD, respectively. One other patient died of pneumonia 21st month after the ESD. There was no recurrent tumor during the mean follow-up period of 23.6 months in this group.
Of the five patients received adjuvant surgery, the average number of dissected lymph nodes was 37.8 (range, 30-47). Only one patient (20%) had tumor cells infiltration in one of the dissected lymph nodes.
4. Discussions
Adjuvant therapy is required for some of the patients with pT1b esophageal cancers after the ESD because of the risks of LNM which is not identified by pre-ESD imaging studies. The risk of LNM for pT1b was reported to be 25.9-53.8% [18-20]. However, these data were from retrospective surgical series. Applying these surgical data to the patients undergoing ESD with the results of pT1b may overestimate the risk of LNM for the following reasons. First, the surgical series included the patients with any nodal status but for the ESD, only patients with negative nodal status were enrolled. Shin et al. reported that the risk of unexpected LNM was only 9.7% (7/72) for the patients with cT1aN0M0 SENs undergoing surgery [21]. Second, the surgical series included SENs with deep submucosal invasion. In this study, the mean depth of tumor invasion in the submucosal layer was 0.79 mm, implying that the patients undergoing ESD with the results of pT1b may have shallower submucosa invasion. Araki et al. reported that the incidence of LNM increased from 13.0% to 58.3% as the depth of tumor invasion in SM layer ranged from <1 mm to >2 mm, respectively [20]. Third, surgically resected specimens were usually cut into 5-mm slices, which was much thicker than the 2-mm slices of ESD specimens [19,20]. Thick sliced specimens may result in under stage of the SEN, leading to overestimation of the rate of LNM for a particular depth of invasion.
A literature review revealed that LV invasion and invasion depth by the tumors were the two common independent risk factors for LNM of SENs [18,22,23]. Miura et al. reported that LV invasion was the most important factor for prediction of LNM [24]. In this study, the mean depth of tumor invasion in the submucosal layer in the patients with positive vertical resection margin was 1.2 mm (range, 0.8- 1.5mm), deeper than those with free margins (0.69 mm, p = 0.025). In other words, positive vertical resection margins indicated not only the possibility of incomplete treatment but also the tumor infiltration to the deeper SM. Therefore, the patients with either one of the two risk factors after the ESD should receive adjuvant therapy.
Either surgery or CRT could be the adjuvant therapy [10,13,25]. Adjuvant surgery resulted in good oncological outcomes but it did cause morbidity and even mortality in the patients. In an abstract form, Muto et al. reported that endoscopic resection combined with prophylactic CRT resulted in a 3-year overall survival of 90.7% for the 87 patients with pT1b with negative resection margin and pT1a with vascular invasion [25]. Ikeda et al. reported that the patients with adjuvant therapy (surgery or chemoradiotherapy) had a better 3-year relapse-free survival rate than the patients without (88% vs. 64%) [13]. However, in this study, whether the patients underwent adjuvant therapy or not were based on the doctor’s discretion or the patient’s preference but not the risk of LNM after the ESD. The patients without risk of metastasis might have received unnecessary adjuvant therapy and the patients with risk of metastasis might be undertreated.
The major limitation of the present study is its retrospective design with a relatively small case number from a single center. Prospective, large-scale studies are required to confirm our observations.
In conclusion, ESD with/without adjuvant therapy based on the pathological findings after the ESD may be an alternative treatment for patients with pT1b SENs but needs further study to confirm.
Competing Interests
The authors declare that they have no competing interests.