1. Background
Functional dyspepsia (FD) has been subclassified into two new disease categories under the Rome III classification criteria: epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS) [1]. Most of FD patients complain various symptoms related to the intake of meals; however, the athophysiology of FD remains poorly defined [2,3]. A number of potentially important abnormalities have been reported in FD patients, including impaired fundic accommodation [4], gastric hypersensitivity to distention [5], abonormal duodenojejunal motility [6], duodenal motor and sensory dysfunction [7], duodenal hypersensitivity [8], hereditary factors [9,10], Helicobacter pylori infection [11] and other infections. Impairment of gastric motility such as gastric emptying is strongly associated with the pathophysiology of FD, one of the most common gastrointestinal disorders [12]. In addition, several studies have reported that gastric motility was also associated with psychogenic factors in FD patient’s [13,14]. We have previously reported that Tmax value as a marker of gastric emptying in PDS patients was significantly greater compared with that of healthy volunteers [15]. We have reported that prokinetics like mosapride citrate improves clinical symptoms by affecting the Tmax value in proton pump inhibitor (PPI)-resistant NERD patients with impaired gastric emptying [16]. In addition, we have also reported that nizatidine administration significantly improved both gastric emptying and clinical symptoms in FD patients with impaired gastric emptying [17]. Therefore, we have considered that Tmax value using 13C-acetate breath test were the useful marker for treatment of FD patients.
In contrast, previous studies have reported that delayed gastric emptying was about 30% of the patients with FD [18-20]. In addition, Kusano et al have reported that rapid gastric emptying might be a more important factor than delayed gastric emptying in patients with FD [21]. Another study has reported that gastric emptying rate (ml/min) at 5min of the patients with FD was significantly higher compared to healthy volunteers [5]. However, early phase of gastric emptying could not be evaluated by Tmax value or T1/2 values. AUC values within 15mins may be important for consideration for etiology of FD patients through evaluating initial gastric emptying within 15 mins. Therefore, in this study, we aimed to determine to investigate whether AUC15 or AUC15/AUCinf values as initial gastric emptying within 15 mins were also associated with clinical symptoms and and depressive state in FD patients.
2. Materials and Methods
2.1 Subjects
Thirty healthy volunteers (Age: 28-52, Sex: M25/F5, BMI: 2.92±0.59), with no clinical history of gastroduodenal disease including symptoms of FD, were recruited. Exclusion criteria included severe heart disease, renal or pulmonary failure, liver cirrhosis, severe systemic illness, history of malignant disease, and erosive GERD. Patients with previous gastroduodenal surgery, duodenal ulcer scars, diabetes mellitus, and recent use of non-steroidal anti-inflammatory drugs, proton pump inhibitors or anticoagulants at endoscopy were also excluded.
Twenty consecutive patients (Age: 35-80, Sex: M3/F17, BMI: 22.34±0.93) presenting with typical symptoms of FD were enrolled after upper gastrointestinal endoscopy. Patients presented with various types of abdominal symptoms including nausea and upper abdominal discomfort, in addition to the four typical upper abdominal symptoms defined by the Rome III criteria [22]: bothersome postprandial fullness, early satiation, epigastric pain and epigastric burning. Dyspeptic symptoms were defined as pain or discomfort in the upper abdomen for the past three months, with symptom onset at least six months prior to medical check-up. Diagnostic criteria for PDS included bothersome postprandial fullness after ordinary sized meals, and/or early satiation that prevented completion of a normal meal, with either symptom occurring at least several times a week. Diagnostic criteria for EPS included all of the following: pain or burning that is intermittent, localized to the epigastrium, of at least moderate severity, and occurring at least once per week.
Written informed consent was obtained from all subjects prior to upper gastrointestinal endoscopy and abdominal ultrasonography for evaluation of dyspeptic symptoms. The study protocol was approved by the Ethics Review Committee of Nippon Medical School Hospital.
2.2 Clinical symptoms
Clinical symptoms of FD were evaluated according to the Rome III criteria [22]. We assessed abdominal symptoms using the modified Glasgow dyspepsia severity score (GDSS) [9, 17, 23, 24], which is based on frequency (never, score 0; on only 1 or 2 days, score 1; on approximately 1 day per week, score 3; on approximately 50% of days, score 4; on most days, score 5), duration (minimun score, 0; maximal score 5), and intensity of symptoms (minimun score, 0; maximal score, 3). Status of depression was evaluated by Self-Rating Questionnaire for Depression (SRQ-D) scores [25].
2.3 Measurement of gastric motility
Sodium acetate (water-soluble) for emptying of liquids was used as tracer (Cambridge Isotope Laboratories, Cambridge, Mass., USA). The liquid test meal consisted of 100mg of 13C-acetate dissolved in 200ml of a liquid meal (Racol, 1 ml/1kcal; Otsuka Pharmacia Company, Tokyo, Japan). Breath samples were collected 0 and 10s and 5, 10, 15, 20, 30, 40, 50, 60, 75 and 90 min after ingestion of the test meal at 10:00 a.m. Patients were instructed not to drink, eat or smoke during the test. Probes were analyzed by non-dispersive infrared spectroscopy (IRIS, Wagner Analyzentechnik, Bremen, Germany). The subject’s own production of 300mmol CO2 per m2 body surface and per hour was set as default. We used an Integrated Software Solutions program to calculate the half gastric emptying time (T1/2) and the lag phase (Tmax; min) as the point of maximum gastric emptying according to Hellmig et al. [26]. The half gastric emptying time (T1/2) represents the time when 50% of the initial gastric content was emptied. A Tmax value of >65 min, representing the mean Tmax in healthy volunteers plus 2SD, was defined to represent disturbances in gastric emptying according to the diagnostic criteria of the Japan Society of Smooth Muscle Research.
2.4 Data analysis
The time plot of pulmonary [13CO2] excretion (%dose/hr) was fitted
to the function:
(% dose / hr) = m × k × ß × e-kt x (1 - e-kt) ß-1
where m is the cumulative [13CO2] recovery at the infinite time, t is in
hours and k and ß are regression estimated constants.
(Cumulative % dose) = m × (1 - e-kt)ß
AUC5 × m x (1 - e-k × 0.08 )ß [T : 5 min = 0.08hr]
AUC15 × m x (1 - e-k × 0.25 )ß [T : 15 min = 0.25hr]
AUC30 ×m x (1 - e-k × 0.5 )ß [T : 30 min = 0.5 hr]
AUC60 × m x (1 - e-k × 1.0 )ß [T : 60 min = 1.0 hr ]
AUC90 × m x (1 - e-k × 1.5 )ß [T : 90 min = 1.5 hr]
AUCinf (AUC8) × m x (1 - e-k × 24 )ß [T : 1440 min = 24 hr]
We determined AUC5 and AUC15 values as the marker of early phase
of gastric emptying based on previous reports [4,5,27-30]. AUC5 value
of > 17.4 and AUC15 value of > 39.6 representing the mean AUC value
of healthy volunteers plus 2SD was defined to represent disturbance in
early phase of gastric emptying.
2.5 Sample size
In our study, we determined the sample size using the PS (Power and Sample size calculations program) software program, a gift from Vanderbilt University. The deviation of the AUC15 value in healthy volunteers was approximately 2.2 (s=2.2). Using the above data and settings of a=0.05, ß=0.80, effect size = 0.639 and an estimated mean AUC15 value of 42.507 (% dose) in patients with FD, thirty healthy volunteers and 20 FD patients were determined to be sufficiency to identify clinically relevant differences.
2.6 Statistical analysis
For the statistical evaluation of group data, Students’ t-test for paired data and an analysis of variance (ANOVA) for multiple comparisons were followed by Scheffe’s F test. The Mann-Whitney U test was used for the analysis of categorical data. The data analysis were performed using a standard software package (SPSS version 13.0, Chicago, IL). A p value of < 0.05 was statistically significant.
3. Results
3.1 Characteristics of FD patients and healthy volunteers
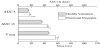
Clinical symptoms including nausea, abdominal fullness, abdominal pain, abdominal discomfort and Glasgow score in FD patients were significantly higher compared to healthy volunteers (Figure 1).
3.2 Comparison of AUC and Tmax values in FD patients and healthy volunteers
Tmax (57.26±3.9) value in FD patients was significantly (p=0.002) greater compared to that (Tmax: 45.53±1.43) in healthy volunteers (Figure 2). To compare AUC90 value with AUC infinity value (AUCinf), we measured AUC90/AUCinf ratio in FD patients and healthy volunteers. Since AUC90/AUCinf ratios in FD patients and healthy volunteers were more than 90% (97.52±0.79, 93.61±2.24, respectively), we calculated AUC5 and AUC15 values in this study as well as in 4hr 13C-acetate breath test. Interestingly, AUC5 (18.41±1.20) and AUC15 (44.17±2.35) values in FD patients were also significantly (p=0.008, p=0.009) greater compared to those (AUC5: 15.18±1.09, AUC15: 35.13±2.22) in healthy volunteers (Figure 2). Then, there was no significant (p=0.714 correlation between Tmax value and Glasgow score in FD patients. Subanalysis of Glasgow score showed that there were no significant relationship (p=0.07, p=0.897, p=0.591) between Tmax alue and nausea, epigastralgia and abdominal discomfort in FD patients.
3.3 Correlation between clinical symptoms and AUC values in FD patients
There were no significant relationship (p=0.230; p=0.259) between AUC5 and AUC15 values and Glasgow score in FD patients. In addition, in this study, to investigate the relationship between initial gastric emptying within 15 mins and abdominal fullness after meals, we compared AUC5, AUC15, AUC5/AUCinf and AUC15/AUCinf values with abdominal fullness. There is no significant correlation (p=0.472,R2=0.0186; p=0.704, R2=0.005) between abdominal fullness and AUC5 and AUC15 values in healthy volunteers (Figure 3A and 3B). AUC5 and AUC15 values in FD patients were not also significantly (p=0.094, R2=0.156; p=0.112, R2=0.142) associated with abdominal fullness (Figure 3A and 3B). AUC5/AUCinf and AUC15/AUCinf values in FD patients were not also significantly (p=0.081, R2=0.168; p=0.104, R2=0.148) associated with abdominal fullness (Figure 3C and 3D). In addition, AUC5/AUCinf and AUC15/AUCinf values in healthy volunteers were not significantly (p=0.838, R2=0.002; p=0.958, R2=0.0001) associated with abdominal fullness (Figure 3C and 3D). We have also investigated the relationship between other clinical symptoms and AUC values in FD patients. There were no significant (p=0.19; p=0.07; p=0.139) relationship between AUC5 value and nausea, epigastralgia and abdominal discomfort in FD patients. In addition, there were no significant relationship between AUC15 value and nausea, epigastralgia and abdominal discomfort (p=0.04, p=0.09; p=0.123) in FD patients. Furthermore, the ratios of the disturbance of Tmax and T1/2 values in FD patients were 33% (10/30) and 30% (9/30), respectively. The ratios of the disturbance of early phase of gastric emptying in FD patients were 23.3% (7/30) and 20% (6/30) for AUC5 and AUC15 values, respectively.
3.4 Correlation between SRQ-D score and AUC values in FD patients
There were no healthy volunteers with depression (SRQ-D score>16). In contrast, four patients were FD patients with depression. We could not find any significant differences in AUC and Tmax values between FD patients with depression and without it. There were significant correlations (p=0.047, R2=0.134; p=0.049, R2=0.131) between SRQ-D score and AUC5 and AUC15 values in healthy volunteers (Figure 4A and 4B). In contrast, AUC5 and AUC15 values in FD patients were not significantly (p=0.608, R2=0.016; p=0.672, R2=0.111) associated with SRQ-D score (Figure 4A and 4B). There were significant correlations (p=0.048, R2=0.133; p=0.047, R2=0.139) between SRQ-D score and AUC5/AUCinf and AUC15/AUCinf values in healthy volunteers (Figure 4C and 4D). However, AUC5/AUCinf and AUC15/AUCinf values in FD patients were not significantly (p=0.702, R2=0.094; p=0.798, R2=0.063) associated with SRQ-D score.
4. Discussion
In the 13C acetate breath test, two popular parameters have been used to quantify gastric emptying rates, namely the time to the maximal excretion (Tmax) and the time to the half (13CO2) recovery T1/2. According to the conventional analysis of Ghoos et al. [31], Tmax value is determined based on a time-profile of the pulmonary (13CO2) excretion rate (%dose/hr) and T1/2 value is based on that of the cumulative amount of (13CO2) recovered in the breath (%dose). Although most studies evaluating gastric emptying in FD patients have reported delayed gastric emptying in a subset of patients, rapid gastric emptying has also been reported [20,21,32]. Sarnelli et al, found rapid gastric emptying for the solid and liquid components of a mixed meal in around 10% [18].Delgado-Aros et al have reported that rapid initial 1hr gastric emptying in 43% of FD patients, and associated gastric hypersensitivity with this type of emptying [33]. In this study, AUC15 value in FD patients was significantly greater compared to that in healthy volunteers. Therefore, it seems to be useful to estimate initial gastric emptying within 15 mins using AUC15 value or AUC15/ AUCinf as well as Tmax value in FD patients. In addition to Tmax value, it is very important for diagnosis of FD patients to evaluate initial gastric emptying in 15 mins postprandial phase. Previous studies have reported that rapid gastric emptying as well as delayed gastric emptying was also linked to functional dyspepsia [21,32]. Gastric emptying and duodenal glucose delivery are closely regulated [27,28]. Early phase of gastric emptying is usually 5-15min in duration and is influenced by intragastric volume and associated with duodenal glucose delivery [27,29,30]. Lunding et al. have also reported that FD patients had significantly fast gastric emptying at 5min compared to healthy volunteers [5]. Therefore, we also investigated AUC15 or AUC15/AUCinf values as initial phase of gastric emptying within 15min in this study. However, further studies will be needed to determine whether AUC15 or AUC15/AUCinf actually reflected on early phase of gastric emptying in FD patients. Tack et al have also reported that approximately 15min are required for maximum gastric relaxation to occur after intake of a liquid test meal [4]. Lee et al have reported that gastric flow into the duodenum including gastric acid inhibits gastric accommodation to a meal [34]. In addition, in a scintigraphic study, early redistribution of the meal to the distal stomach or accelerated gastric emptying in the postprandial period was associated with symptoms of early satiety [35]. Considering previous reports, greater AUC5 and AUC15 values compared to healthy volunteers may be a pivotal factor for interaction between gastric accommodation and early phase of gastric flow into the duodenum.
Next, we compared initial gastric emptying within 15 mins with clinical symptoms in FD patients and healthy volunteers. However, there was no significant relationship between initial gastric emptying at 5min and 15min and abdominal fullness in both of FD patient and healthy volunteers. Most studies following the Rome II criteria have failed to find a good correlation between clinical symptoms and gastric emptying [36].We have first reported the association between abdominal fullness and initial gastric emptying within 15 mins in healthy volunteers and FD patients.
Studies have shown that the prevalence of psychological disorders is significantly higher in patients with FD than in the general population [37,38]. Lee et al have reported a significant correlation between clinical depression and FD [37]. We have also reported that SRQ-D scores in FD patients are relatively higher than that in healthy volunteers. In addition, previous studies have reported that psychogenic factors such as depression are associated with gastric emptying [13,14]. Then, Kusano et al have reported that early phase of gastric emptying plays important roles in FD patients as well as entire gastric emptying [21,32]. Considering these reports, it warrants further exploration to clarify the precise mechanisms underlying disturbed gastric emptying in initial postprandial phase within 15 mins and depressive status in FD patients. Therefore, we compared depressive state with early phase of gastric emptying in healthy volunteers and FD patients using SRQ-D score. However, there was no significant relationship between initial gastric emptying within 15 mins and depressive state in FD patients. Therefore, further studies will be needed to clarify whether psychological disorders such as depression can modulate initial and late phase of gastric emptying in healthy volunteers.
The present study has some limitations. In this study, we compared AUC values in FD patients with healthy volunteers in small sample size. Another limitation of this study was that the precise physiological mechanisms underlying disturbed gastric emptyng in initial postoprandial phase within 15 mins and depressive status in FD patients remains unclear. Taken together, impairment of initial gastric emptying within 15 mins may play critical roles in functional dyspepsia. Further studies will be needed to clarify whether AUC15 or AUC15/AUCinf reflected on early phase of gastric emptying.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Shimpuku M, Yamawaki H, Kodaka Y and Kawagoe T contributed substantially to data acquisition. Sato H contributed towards statistical analysis of the data and interpretation of results. Inamori M, Sakamoto C, Suzuki H and Saitow F cotributed towards direction of the study. Finally Shimpuku M, Futagami S and Gudis K worked with writing the manuscript.