1. Introduction
Breast sarcomas are an etherogeneous group of mesenchymal derived malignancies that occur for less than 1% of all breast malignancies and less than 5% of all sarcomas [1].
The Stewart-Treves syndrome is a secondary form of breast sarcoma and it is a rare phenomenon which has been reported to occur in approximately 0.9 out of 1,000 cases [2]. The syndrome is associated with a history of radiation therapy - Radiation-associated angiosarcoma (RAA) - or with chronic lymphedema after axillary lymph node dissection for mammary carcinoma of the breast.
The incidence of secondary angiosarcoma arising in the irradiated breast after breast conservation therapy (BCT) concerns mainly women treated with partial mastectomy and radiotherapy.
A primary risk factor for post-mastectomy upper extremity angiosarcoma has been detected in the lymphedema of the ipsilateral arm.
In such cases the role of radiotherapy (RT) is not direct but it facilitates lymphatic stasis. Is still un-known whether post-BCT angiosarcoma of the breast is induced by edema or by radiation or is still unknown it has a multifactorial origin. The reported incidence of post-RT breast edema is from 0% to 100% in patients with BCTassociated angiosarcoma.
The rate of breast edema may be underestimated since all patients treated with BCT have some cas-es of edema, perhaps subclinical.
It has been established that radiation-induced sarcomas develop in a period up to 10 years after the treatment. The amount of time between the breast cancer treatment and the diagnosis of BCTassociated angiosarcoma is shorter, approximately 4 to 7 years.
Some cases of angiosarcoma arising in a breast after partial mastectomy not followed by RT are also been described, so the radiation exposure alone is unlikely to be considered the only cause [3].
Initial signs of RAA may be subtle and difficult to identify.
Patients may have skin with faint purple discoloration or scar change.
It often occurs an underlying lesion, but not always. Contrary to primary breast angiosarcomas, which more frequently affect young women, age in RAA ranges widely from 46 to 87 years, with an average of 70 years [4].
2. Case Presentation
We report a rare case of 61 year female breast cancer.
In 2007 the patient underwent a mammography that showed breast left nodules and a lymphadeno-pathy. In July 2007 a left breast quadrantectomy with omolaterallinpho adenectomy was performed and histologycal exams showed breast adenocarcinoma (pT1a; pN1 (1/12); M0; G2; ER: 75%; Pgr: 80%; Her2: neg).
After surgery, a chemotherapic treatment was performed according to FEC schedule (5-Fluorouracil, Epirubicin, Ciclophosphamide) for six cycles, overall well tolerated, followed by ra-diotherapic treatment according to schedule: 50 Gy in 25 treatments over 5 weeks.
All the clinical and instrumental controls were negative until September 2014, when during a routine control, it was showed a periareolar left breast iperemic area, that was later confirmed by ecothomography.
A biopsy was performed in October 2014 and it showed a histological aspect of breast angiosarco-ma. In November 2014 a left breast mastectomy was performed and it showed: breast with fusatemesenchimal cell dermic proliferation. 5/10 HPF mitosis. The tumor was extended in the nipple area and focally in the breast parenchyma. All this data suggested a well differentiated angiosarcoma diagnosis.
Considering the patient performance status sec ECOG (PS1) and the well differentiated tumor cell, the patient was recommended to do intensive follow-ups until July 2015.
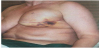
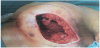
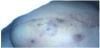
In June 2015 there was clinical evidence of a brown area around the scar (Figure 1) treated with surgical exeresis and left breast prosthesis substitution (Figure 2). The histological exam of this necrotic area confirmed angiosarcoma relapse, G3, Ki67>50%. A stadiation CT was negative, but considering the histological exam, the high risk of metastasization and the persistence of red skin lesions (Figure 3), we decided to treat the patient with “neoadjuvant” chemoterapic approach and a possible successive local treatment.
After a central venous catheter implantation, the chemoterapic schedule given to the patient was Docetaxel 75 mg/m2 g1 + Gemcitabine 1000 mg/m2 g1,8 q21 from July to November 2015, for 6 cycles.The treatment was complicated by neutropenia (G3/G4), liver toxicity (G2/G3), asthenia (G2), haematological toxicity (G2), according to Common Terminology Criteria for Adverse Events (CTCAE) with concomitant complete objectiveclinicalresponse.
After a central venous catheter implantation, the chemoterapic schedule given to the patient was Docetaxel 75 mg/m2 g1 + Gemcitabine 1000 mg/m2 g1,8 q21 from July to November 2015, for 6 cycles.The treatment was complicated by neutropenia (G3/G4), liver toxicity (G2/G3), asthenia (G2), haematological toxicity (G2), according to Common Terminology Criteria for Adverse Events (CTCAE) with concomitant complete objectiveclinicalresponse.
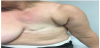
From December 2015 the patient is still disease-free (Figure 4), with a disease free survival of 34 months. She also continues to carry out follow-up.
3. Discussion
Radiotherapy has been found to be a significant risk factor associated with secondary soft tissue sarcomas, in particular with angiosarcoma [5]. RABA is typically a late complication of adjuvant radiotherapy. The median latency is 7.5 years, but there is considerable variation in the time to presentation, ranging from 1 to 26 years [6]. Age, advanced stage and grade 3 of tumor seem to be poor prognostic factors. To date there is no consensus of optimal treatment of RABA. Current rec-ommendations are derived from small retrospective case reviews and extrapolated from non-breast soft tissue sarcoma studies [7]. There are limited evidences to suggest that neoadjuvant chemotherapy produces a survival benefit in RABA. Targeted therapies may offer an alternative treatment in patients with progressive disease. In particular, the tyrosine kinase inhibitor pazopanib demonstrated activity in both locally advanced and metastatic angiosarcoma [8].
Ongoing clinical trials using combinations of VEGF inhibitors and chemotherapy may provide future avenues of treatment for this difficult-to-treat disease.
4. Conclusion
Breast Angiosarcoma is more prevalent in those cases treated with radiotherapy, occurring especially adjacent to the radiation field. Because of the increasing use of breast conservation therapy for breast cancer, there are an increased numbers of cases reported in the literature but few of these are treated with a neoadjuvant approach [9]. Despite breast angiosarcoma remains a disease with difficult management,our case report demonstratesthatneoadjuvant chemotherapy may have a role in downsizing locally advanced disease although it has not proven effect on survival yet.
Competing Interests
The authors declare that they have no competing interests.