1. Introduction
Postpartum uterine pathology is a common cause of morbidity and rarely, mortality in women of reproductive age. It is therefore paramount that clinicians are able to select the most appropriate imaging modality in order to aid accurate diagnosis and subsequent treatment of the postpartum patient.
It cannot be denied that a thorough history from and physical examination of the postpartum patient will more often than not provide the diagnosis, however confirmation is usually obtained through imaging. Portable and departmental ultrasound scanning has become an increasingly utilised investigative tool in the diagnosis of puerperal complications. The safety profile and patient acceptability rates of both transabdominal and transvaginal ultrasound scanning often render it the first-line imaging modality of choice when postpartum pathology is suspected. In contrast to MRI ant CT scanning, the accessibility and relative low cost of ultrasound scanners have allowed clinicians other than radiologists to undertake diagnostic imaging examinations. As a real-time imaging method, it can not only be used as an adjunct to bimanual examinationbut also utilised in the guidance of therapeutic procedures, such as evacuation, aspiration and drainage procedures. Ultrasound also negates the need for intravenous contrast material (often required to produce optimal CT or MRI images) which may interrupt breastfeeding; such agents are known to be excreted in small quantities in human breast milk. The main disadvantage of ultrasound is that it is operator dependent, and when patient body habitus is increased or bowel gas is present, deeper structures are difficult to assess. In these scenarios secondary imaging with CT or MRI may be required for further evaluation of suspected pathology.
It must be emphasised however, that an understanding of the normal appearance of the postpartum uterus is a prerequisite for the accurate diagnosis and management of puerperal pathology, no matter which imaging modality is used.
2. Common Indications for Postpartum Imaging
There are a number of possible indications for pelvic imaging, most commonly suspected retained products of conception or pelvic sepsis, both of which often present with excessive or erratic bleeding.
Primary postpartum haemorrhage (PPH) is traditionally defined as the loss of at least 500 ml of blood from the lower genital tract within 24 hours of delivery,or any blood loss that results in maternal haemodynamic compromise [1-4]. Although the most common cause of primary PPH is uterine atony, care must be taken to exclude retained products of conception (placenta and membranes) or intrauterine blood clots as an additional or primary cause [4]. Clearly any offward radiological assessment (e.g. CT or MRI scanning) is rarely indicated in this emergency setting, yet bedside ultrasound scanning can be a useful diagnostic adjunct to clinical examination in this scenario. In more complex cases, where intraabdominal haemorrhage is suspected, CT has been proposed as a superior mode of imaging, permitting localisation of arterial bleeding sites and haematomas, along with vascular mapping, which is necessary should endovascular embolisation therapy be required [5].
The occurrence of increased or abnormal genital tract bleeding occurring between 24 hours and 12 weeks postpartumis defined as secondary PPH, and is often a sign of underlying endometritis or retained products of conception [4,6]. Abnormal bleeding up to six weeks is the more commonly used definition of secondary PPH in the UK and in developed countries 2% of postnatal women are admitted to hospital with such symptoms [7]. Up to half of these women will undergo surgical evacuation in accordance with the guidance published by the Royal College of Obstetricians and Gynaecologists, who recommend surgical treatment when there is excessive or ongoing bleeding, irrespective of ultrasound findings [4,7]. This is based on the understanding that although pelvic ultrasound can aid the exclusion of retained products of conception, the appearance of the immediate postpartum uterus can be unreliable [8,9] no matter which imaging modality is used. It is our belief that a better understanding of postpartum ultrasound findings could enable more accurate identification of women requiring surgical intervention, with consequent reduction in surgical complications, radiation exposure, and cost to healthcare services.
Occasionally the request for postpartum imaging may follow the clinical finding of a pelvic mass, or suspected adnexal mass accident. It is unlikely that such masses would not have been detected during antenatal screening scans however the discovery of ovarian cysts postpartum is not unheard of. In this setting, should there be diagnostic uncertainty as to the nature of the cyst, an MRI scan may be a useful secondary imaging tool following the initial ultrasound scan.
3. The Normal Postpartum Uterus
Immediately following delivery, the action of oxytocin (endogenous or synthetic) onthe uterus causes it to undergo a rapid involution. The average mass of the term uterus post-delivery is 1000g, at which time the uterine fundus can oftenbe palpated at the level of the umbilicus. By day seven postpartum, the uterine mass has halved, and a further seven days later, the uterine size decreases such that is no longer palpable abdominally, returning to the true pelvis. After six weeks the uterus has decreased to 50-100g, a size consistent with a non pregnant state, however the final uterine size will remain larger than the original nulligravid state. In contrast to the gradual recovery of the uterus, the postpartum endometrium regenerates at a rapid pace. By the seventh postpartum day, endometrial glands are already formed, and after two weeks the endometrial lining is almost completely restored throughout the uterine cavity, with the exception of the placental bed site [10].
The vast majority of this knowledge was obtained historically from histological analysis of post mortem specimens, when puerperal maternal death rates were higher [11] fortunately, in more recent times, uterine imaging (specifically ultrasound) has been the mainstay in furthering our knowledge of postpartum uterine physiology and appearances.
4. Radiological Appearances of the Postpartum Uterus
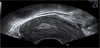
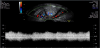
There is abundant data and description regarding the ultrasound appearances of the process of normal uterine involution in the literature, (Figure 1) [12] however very few studies exist where CT or MRI have been used to image the normal postpartum uterus. Althougha large proportion of these studies span a number of decades, there is a general consensus that a significant overlap between the normal and abnormal uterus exists, across all modes of imaging [9, 12-14].
A more recent study of the postpartum uterus assessed both the anteroposterior (AP) diameter of the uterus and the endometrial thickness (postpartum uterine lining), and the findings indicate that mode of delivery and gestational agecan affectthe rate of uterine involution. For example, the reduction in endometrial thickness over the postpartum course following a term vaginal delivery was found to be significantly greater than that following term caesarean section; additionally, the decrease in the AP diameter of the uterus was less after a preterm delivery versus a term delivery. Although based on a small number of cases, this study supports the theory that the process of uterine involution will vary with both mode and timing of delivery [15].
Despite numerous imaging studies assessing uterine involution, there are conflicting data describing the typical radiological appearances of the uterine cavity and its contents postpartum, and also the relevance of such findings to clinical practice. In order to aid understanding of the physiological and pathological characteristics of the postpartum uterus, radiological assessment of the uterine cavity can be broadly divided into two categories: (1) the immediate post partum period i.e. within the first 24 hours, and (2) 24 hours postdelivery through to the end of the puerperium.
Despite numerous imaging studies assessing uterine involution, there are conflicting data describing the typical radiological appearances of the uterine cavity and its contents postpartum, and also the relevance of such findings to clinical practice. In order to aid understanding of the physiological and pathological characteristics of the postpartum uterus, radiological assessment of the uterine cavity can be broadly divided into two categories: (1) the immediate post partum period i.e. within the first 24 hours, and (2) 24 hours postdelivery through to the end of the puerperium.
5. Radiological Appearances Immediately Postpartum (within 24 hours)
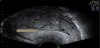
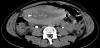
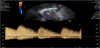
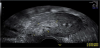
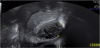
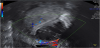
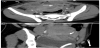
The presence of intrauterine blood clots, debris, and/or fluid is common following vaginal delivery, and will appear as areas of increased echogenicity on ultrasonographic assessment. (Figure 2) Should a CT scan be undertaken, a central area of low-attenuation will be revealed, however in the presence of fresh bleeding from the placental bed these clots will appear as hyperattenuating material (Figure 3) [5]. The presence of intracavity gas can be considered a normal postpartum finding in the absence of abnormal symptomatology, and will appear as gas bubbles on CT, or foci of echogenic shadowing on ultrasound.
The volumes of intracavity material have also been studied: in a prospective observational study of 94 women, Deans et al. sought to establish if there was any correlation between transabdominal ultrasound findings and patient morbidity in the first 24 hours postpartum [16]. Unexpectedly large volumes of echogenic material were revealed within the uterine cavity, in particular within the lower segment of the uterus, where mean volumes were as great as 54.8cm3. However, there appeared to be no correlation between the presence of this material and the development of postpartum morbidity, such as pyrexia, PPH or prolonged hospital stay. This suggests that the presence of large volumes of intrauterine echogenic material in the first day post-delivery can be accepted as normal.
In a similar study, immediate ultrasonographic assessment was undertaken following placental delivery, but with concomitant surgical curettage of the uterine cavity (within two minutes of the scan) [17]. Following histological assessment of the intrauterine material, the sensitivity, specificity, positive and negative predictive value of ultrasound in detecting retained products in their study was 44%, 92%, 58%, and 87%, respectively. Of those patients with histologically confirmed retained products of conception, a large proportion in fact had a normal endometrial cavity on ultrasound scan (37.5%). The remainder had either an echogenic mass, a heterogeneous mixed density mass, or intrauterine fluid alone. The vascularity of these intrauterine masses was however not assessed. Thus it can be concluded that in the absence of colour Doppler assessment, the appearances of retained products immediately following delivery are highly variable and cannot be correlated with a need for intervention.
6. Radiological Appearances after the first 24 hours Postpartum
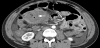
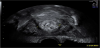
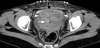
As previously mentioned, the postpartum uterus is often found to contain an accumulation of clinically insignificant debris and fluid, mainly in the lower segment initially and then within the whole uterine cavity by the middle of the puerperium [12]. Various studies have also suggested that the presence of intracavity gas, visible on imaging for several weeks postpartum (Figure 4), can also be accepted as a normal puerperal finding [18,19].
In a recent observational study a small group of women were systematically scanned at weekly intervals postpartum, starting from week one up until week three [9]. These ultrasound assessments revealed that in women with normal postpartum bleeding, there was an echogenic mass in 51% on day seven, in 21% on day 14 and in 6% on day 21. They found no difference in either the heaviness or bleeding duration between women with and without an echogenic mass at each of these three scans. Thus, the authors hypothesised that either an echogenic mass does not always represent retained products of conception, or that products of conception are commonly retained and are therefore of little clinical significance in many cases. However, Doppler assessment of these products did not appear to be undertaken, nor was there any clarification by the authors as to whether the description of an echogenic mass also included mixedecho patterns, a finding which other studies have suggested is an insignificant postpartum occurrence [20]. Care must therefore be taken to interpret ultrasound scan findings in this clinical context; the specific finding of an echogenic mass in the setting of secondary PPH is likely to be associated with retained placental tissue and requires surgical intervention, whereas mixed-echo patterns may not, and can be managed expectantly, with early resolution of symptoms [20].
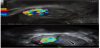
The addition of colour Doppler assessment at ultrasound scan can aid identification of placental remnants [21]. A cross-sectional study of 385 postnatal women revealed areas of enhanced vascularity in 32 women (8.3%), and 26 women (6.75%) had retained placental products on scan. Although no comment was made regarding patient morbidity, a high incidence of histological confirmation was obtained following surgical curettage (19 of 20 cases), suggesting that the use of colour Doppler may be of practical diagnostic value [21]. The following images demonstrate the use of colour Doppler in aiding the diagnosis of retained products of conception, later confirmed on histological analysis.
7. Rare Postpartum Pathology and Imaging Findings
Less common postpartum ultrasound findings are summarised below.
7.1 Caesarean Section Associated Findings
Globally rising rates of caesarean section delivery have led to an increased understanding of normal and abnormal post-caesarean imaging findings. Following an uncomplicated procedure, ultrasound will identify the uterine incision as an iso- or hypoechoic region when compared to myometrium, and when imaged in the sagittal plane on transvaginal scan, is centrally located between the uterus and bladder [22-24]. Depending on probe orientation, the uterine sutures can be identified as linear or point-like hyperechoic foci, and small haematomas (<15mm) along the suture line can be considered as normal [22-24]. On CT, the incision site will demonstrate transverse focal thinning; as the transverse incision will be parallel to the axial imaging plane, sagittal reformatted CT images are better in demonstrating the area of decreased attenuation that will be present in the lower uterine segment (Figure 10) [5]. The images can be further enhanced through intravenous contrast administration, however resulting image quality will always be limited due to the relative fluid overload associated with pregnancy and surgery [22].
If adequate haemostasis has not been achieved intraoperatively, the immediate post-operative period may be complicated by the formation of a bladder flap haematoma. During a lower segment caesarean section, the visceral peritoneum is incised between the uterus and bladder, and reflected inferiorly. It is in this space that a haematoma may form, and on ultrasound will be seen as a non-vascular mass of mixed echogenicity, anterior to the uterus and posterior to the bladder, and has been described as a ‘bladder flap’ haematoma in the literature [5,22]. (Figures 11 & 12) These may or may not be contained by the overlying peritoneum, and in the latter scenario will lead to the detection of haematoperitoneum on scan. At CT, the bladder flap haematoma will appear as a slightly hyperattenuating area in the same location (Figure 13), with possible mass effect [5,22]. The presence of such a haematoma is generally considered to be of little clinical significance if they are below 4 cm in size [22].
7.2 Pelvic sepsis
Few studies exist which specifically report the imaging findings expected in postpartum endometritis, however in contrast, cases of pelvic sepsis with underlying abscesses are frequently described. [22,25,26] Superimposed infection of a pelvic or bladder flap haematoma may lead to the formation of a pelvic abscess, the diagnosis of which can be achieved with ultrasound. The abscess will appear as a well-circumscribed fluid collection with or without internal septations, containing internal debris. The presence of gas, seen if gas-producing organisms are present, will cause multiple highly echogenic foci, leading to dirty posterior shadowing on scan [22]. CT scan will reveal a gas containing fluid collection, again with or without internal septations, and will be rim-enhancing [22].
7.3 Arteriovenous malformations
With now over 200 cases reported in the literature, uterine arteriovenous malformations (AVMs) are not as rare as once thought, following the first reports almost 90 years ago [27]. These pelvic lesions are an acquired anomaly and an association with trophoblastic disease, pelvic surgery (e.g. myomectomy), endometrial curettage, uterine malignancy and caesarean scar pregnancy have all been reported. A congenital aetiology has been postulated, particularly in cases where there is multiorgan involvement and the presence of multiple AVMs. They are most prevalent in women of reproductive age, rarely occurring in the nulligravid. Thus it has been hypothesised that pregnancy contributes to the pathogenesis of uterine AVMs [28] where necrosis of chorionic villi leads to the incorporation of venous sinuses into areas of myometrial scarring.
The clinical presentation may be with either primary or secondary PPH, and rarely, a pulsatile pelvic mass. The volume of blood loss can be extensive and swift, leading to rapid haemodynamic compromise. Although the current gold standard diagnostic test is pelvic angiography, the use of Doppler ultrasound can successfully identify these vascular lesions. The typical appearance is of a highly vascular localised area within the myometrium. (Figure 14) Pulsed Doppler evaluation will usually reveal a low-resistance blood flow with a broad waveform, high peak velocities and signs of turbulence. [28]Treatment is typically with selective embolisation of the feeding vessel, and less often surgical excision of the lesion. Both CT and MRI can be useful in the identification of feeding vessels; contrast CT will reveal the numerous tortuous vessels coursing through the parametrium and uterine wall, and MRI will demonstrate a localised disruption of the uterine junctional zone with multiple serpiginous signal voids [26].
7.4 Uterine dehiscence and rupture
An incomplete rupture of the uterine wall, where by the serosal layer of the uterus remains intact is classed as uterine dehiscence. Should this layer also be disrupted then the more serious complication of caesarean delivery, uterine rupture, has occurred. Uterine dehiscence poses a diagnostic challenge due to the significant overlap with the normal uterine appearances post caesarean section. The presence of a bladder-flap haematoma greater than 5 cm in size identified on either ultrasound or CT should raise the suspicion for uterine dehiscence [22]. In this clinical setting, an MRI scan may be superior for confirming the diagnosis in view of its enhanced soft-tissue contrast and multiplanar capability, enabling depiction of an intact serosal layer [22]. In contrast, the diagnosis of uterine rupture can be more readily achieved on ultrasound or CT scan. Focal disruption of the myometrium, the presence of gas extending from the endometrial cavity to the parametrium, and the addition of haematoperitoneum are imaging findings that will typically provide the diagnosis (Figure 15a and Figure 15b) [5,22].
8. Conclusions
Ultrasound can be considered the first-line imaging modality of choice in the assessment of puerperal uterine pathology, providing a safer and more acceptable experience for the patient, and an accessible and relatively inexpensive option for the clinician. In the event of rarer postpartum complications with consequent diagnostic uncertainty, CT is often used as a secondary imaging adjunct. However in order to obtain more detailed assessment of the uterine soft tissues (e.g. should dehiscence be suspected) MRI is the modality of choice. It is, therefore, key that the most appropriate imaging modality is chosen for the accurate assessment and characterisation of postpartum uterine pathology, thus ensuring timely and correct treatment of the puerperal patient.
Competing Interests
The authors declare that they have no competing interests.