1. Introduction
The El Cajete Series is the youngest product of the well-known, silicic, resurgent Valles Caldera of the Jemez Mountains in northern New Mexico. The Jemez Volcanic Field evolved over the past 16 Ma [1] . The magma system of the Valles caldera complex formed at the intersection of Jemez Lineament and Rio Grande Rift, produced two major caldera-forming eruptions, which formed the lower and upper Bandelier tuffs at, respectively, 1.61 and 1.22 Ma [2-7] . Following the eruption of upper Bandelier Tuff and the formation of the Valles Caldera, a series of rhyolite domes, the Valles Rhyolite Formation, erupted from ring fracture vents in the Valles Caldera at 1.1 Ma and extended over a 0.7 m.y. interval [8,9]. The most recent eruptions of the Valles Caldera produced the South Mountain Rhyolite (SMR), 507 Ma [2] to 521 Ma (39Ar /40Ar) [10] , and the El Cajete Series (ECS), 50 to 60 ka [11-14] at the southwestern moat of the Valles Caldera (Figure 1). New 40Ar/39Ar sanidine single crystal ages indicate that ECP and BAT erupted at 74.4±1.3 ka and BB erupted at 68.3 ± 1.5 ka [15] .
South Mountain rhyolite crops out along the southern margin of the caldera. The three El Cajete members were the products of a long eruptive sequence. The stratigraphic relationships among the ECS are, from the oldest to the youngest, El Cajete Pumice Fall (ECP), Battleship Rock Tuff (BAT), and Banco Bonito Lava Flow (BB) [16-18] . ECS has lower silica rhyolite (SiO2 72.04-75.29 wt%) compared with SMR (SiO2 76.3-79.25 wt%) (Table 1) (Supplementary File). The three members show only slight compositional variation. The El Cajete pumice (ECP) erupted first, forming Plinian style pumice fall and associated pyroclastic flow deposits [16] has lower SiO2 (72.28- 73.88); Banco Bonito lava (BB) the youngest member in the series [17] has higher SiO2 (73.08-75.94).
The energy and the areal distribution of ECP were the largest in the whole series. The newest estimated volume of the ECP is about 7.6 km3 dense rock equivalent (DRE) [18] (much larger than the previous 1.3 km3 DRE [16,19]). Battleship Rock Tuff (BAT) (DRE ~ 3 km3) was formed by a series of pyroclastic flows erupted from a vent near El Cajete Crater [20] . After the eruption of the BAT, there was a lull of around 6 ka and the surface of BAT was eroded [15] . The BB later filled some of these gullies over BAT [16,18,19,21]. The BB is a porphyritic glassy flow that erupted from a vent located 1 km northwest of the El Cajete Crater. According to the extent and thickness of the flow, the volume of the Banco Bonito is estimated 4 km3 DRE [19] .
This paper discusses geochemical variation within the ECS. Using phenocryst compositions and geothermometry and geoberometry, this paper will also discuss the possible geothermal condition of ECS and SMR. ECS formed from a deeper and less silicic chamber. There are reversed compositional gradients in the three members. The early eruption has a lower silica composition and the latest one has the highest silica. Trace elements show the more detailed change. Eruption dynamics caused the ECS to be erupted in a reversed compositional sequence.
This paper discusses geochemical variation within the ECS. Using phenocryst compositions and geothermometry and geoberometry, this paper will also discuss the possible geothermal condition of ECS and SMR. ECS formed from a deeper and less silicic chamber. There are reversed compositional gradients in the three members. The early eruption has a lower silica composition and the latest one has the highest silica. Trace elements show the more detailed change. Eruption dynamics caused the ECS to be erupted in a reversed compositional sequence.
2. Sampling and Methods
Forty one rock samples were collected from three different units: Bandelier tuff (4), South Mountain Rhyolite (SMR) (10), and El Cajete Series (ECS) (27). Within the ECS, nine samples were taken from El Cajete Pumice Fall (ECP) deposit (all pumice), twelve from the Battleship Rock Tuff (BAT) (nine pumice and three welded tuff), and seven from the Banco Bonito Lava Flow (BB) (all vitrophyre).
Electron microprobe analyses were made of all phenocryst phases and some glass. Analyses were performed on a Cameca Camebax electron microprobe at Baylor University and JEOL JXA8900 at University of Nevada Las Vegas. Operating conditions were: 15 kV accelerating voltage, emission current of 10 nA for feldspar and 15 nA for other minerals, spot sizes are 10 μm for feldspar and 3 μm for other minerals. Standards were appropriate silicates and oxides.
Whole-rock major and selected trace elements were analyzed at Baylor University by wavelength-dispersive XRF. A tungsten-carbide shatterbox was used to powder the samples. Six grams of the powder were combined with one gram of bakelite and then compressed into a pellet. Pellets were put into an oven to heat for half an hour at 115 oC. The average standard deviation for BBB is mostly less than 2% for major elements and less than 10% for trace elements. REE INAA analysis was performed by Activation Laboratories, Canada.
3. Results and Discussion
3.1 Petrography
3.1.1 El Cajete Pumice Fall (ECP)
ECP pumice contains phenocrysts of plagioclase 10-15%, quartz 5-8%, amphibole 2-3%, and biotite 3-5%. The plagioclase is white, about 1-2 mm in length, and mainly subhedral. The quartz is colorless, granular, and 1-2 mm in diameter. Amphibole is needle-shaped, euhedral, black or dark green color and about 1-3 mm in length. Biotite is sheet-shaped, black color, and 1-2 mm in diameter. Because the pumice is very soft, no thin sections were prepared for them.
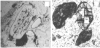
Partly resorbed plagioclases in ECP contain glass. The glass in plagioclase contains gas cavities (Figure 2a). A SEM image shows the boundary between plagioclase and glass in Figure 2b. There are large gas cavities in the pumiceous glass and some gas bubbles in plagioclase. The gas bubbles in glass within plagioclase show some connection channels with the surrounding glass (Figure 2b).
3.1.2 Battleship Rock Tuff (BAT)
BAT consists of three zones: a lower unwelded zone, middle welded zone, and upper unwelded zone. In the lower and upper zones, unsorted pumices are scattered in an ashy matrix. The color of the Battleship Rock tuff pumices is usually yellowish brown, and the core of large pumices (10-20 cm in diameter) is gray. The phenocrysts in the pumice are plagioclase 5-8%, hornblende 1-2%, biotite 1% +, clinopyroxene <1%, orthopyroxene <1%, magnetite and ilmentite <1%, quartz 1% +, and a few grains of sanidine.
The plagioclases are mainly subhedral, 1-3 mm, compositionally zoned. Most plagioclases have normal zonation, some with reversezoned rims. The hornblendes are euhedral, 0.1 - 0.3 mm, most of them occurring as isolated grains, but some occur as mantles over clinopyroxene and biotite. Clinopyroxenes are mainly euhedral, 0.1 - 0.3 mm (Figure 3a). Most biotites have hornblende overgrowths (Figure 3b). There are plagioclase, biotite, hornblende aggregates in the rock. The crystal size of plagioclase (< 1 mm) in the aggregates is smaller than most isolated plagioclase grains.
3.1.3 Banco Bonito Lava Flow (BB)
BB is largely vitrophyric. The color of the rock is glassy black. The phenocrysts are flow aligned and make up about 16 volume percent of the rock. The phenocrysts are plagioclase 8-10%, quartz 2-3%, biotite 1% +, clinopyroxene and orthopyroxene < 1%, hornblende 2-3%, and <1% opaque minerals.
The plagioclases are mostly subhedral. Most of the plagioclases are normally zoned, some with reverse-zoned rims. Some of them have resorbed cores but glass-free rims (Figure 4A). The resorption of plagioclase in this unit is less than in ECP and BAT. Clinopyroxenes are usually subhedral and larger than orthopyroxene. Orthopyroxenes are usually euhedral to subhedral, scattered in glass. Biotites are euhedral to subhedral, most of them with hornblende overgrowth. Most hornblendes are usually euhedral and occur as isolated grains in glass (Figure 4b). Quartz generally is anhedral, with resorbed edges.
Crystal aggregates are distributed in BB. Within the aggregates, hornblende mantles the biotite and pyroxene. The plagioclases have cumulate texture. The grain size of minerals in aggregates is smaller than that of single grain.
3.1.4 South Mountain rhyolite (SMR)
SMR is white and porphyritic. Phenocrysts of plagioclase, sanidine, quartz, and biotite are set in a glassy groundmass. The ratio of phenocrysts to glass is about 40:60. There are about 25-30 vol.% feldspars in the bulk rock. Granular quartz is gray to light-amethyst in color, about 10-15 vol.%, and about 1-2 mm in diameter. The schistose biotites are black in hand specimen and less than 2-3 vol.%.
3.2 Geochemical variation in ECS
The El Cajete series (ECS) has SiO2 < 75 wt%, and shows a general increasing trend for SiO2 from early eruption ECP, to BAT, to later BB. On Harker diagrams (Figure 5), Al2O3, FeO, CaO show negative trends while MgO, Na2O and K2O show positive trends with increasing SiO2 for ECS. The aluminum saturation index (Al2O3/(CaO + Na2O +K2O)) shows that the ECS is mainly a peraluminous series. Trace elements plotted versus SiO2 show some evolutionary trends with increasing silica (Figure 6). In the El Cajete Series, Sr, Ba, and Zr show negative trends with increasing SiO2; Rb, Nb and Y have slight positive trends. SMR has different trends compared with those of the ECS.
Rare Earth Element analyses were made for eight samples by INAA (Table 1) (Supplementary File). Within the ECS, REE variation is not so obvious. ECP and BB are similar; BB is slightly richer in HREE. BAT is lower in La, and Yb (Figure 7). The Eu negative anomaly in BAT is a little smaller than ECP and BB. Therefore, BAT seems slightly less evolved than ECP and BB. The two SMR samples show higher HREE and a stronger Eu anomaly. The BAT is slightly depleted in Hf, Th, Y, and Nb compared to the rest of ECS.
The ECS shows reversed compositional gradients within the three members. The common pattern of such eruptive sequences is usually to show a progression from most evolved to least evolved magma. Sr, Ba, and Zr would show roofward depletion. HREE and HFSE are usually concentrated roofward in zoned eruptions [22] . The earliest eruption (ECP) has lower silica and the latest (BB) has the highest (Figure 6). Trace elements show more detailed change. BB has the lowest Sr, Ba and Zr contents (Figure 8). Incompatible elements (REE, Th, Nb, and Hf) in the BAT are the lowest. Thus, the ECS differs from normal eruptive sequences.
South Mountain Rhyolite and Bandelier Tuff (BT) samples are also plotted on the Harker diagram for comparison (Figure 5). SMR forms trends distinct from those of the ECS. With increasing SiO2, Al2O3, FeO shows negative trends and K2O shows a positive trend with different slopes for SMR samples compared to ECS; MgO, CaO, and Na2O for SMR show reverse trends compared to ECS. The trends of the Bandelier Tuff are similar to the SMR. Trace elements for the Bandelier Tuff and SMR are also plotted on Figure 6. Their trends are very similar except that the Bandelier Tuff has higher Zr than SMR. Therefore, the SMR may be more closely related to the Bandelier Tuff magmatic system, which formed the caldera. According to the geochemical data, SMR and ECS are two different magmatic systems, showing contrasting evolutionary trends.
3.3 Resorption texture and hornblende overgrowth in ECS
Plagioclase is resorbed in all three members of ECS. Resorption is greater in plagioclase of ECP and less in BB. Magma mixing is considered to be a major cause for resorbed volcanic plagioclase, but plagioclase resorption can also occur if plagioclase crystallized at high pressure has become unstable during decreasing pressure accompanying magma ascent [24-26] . Experimental data indicate a systematic increase in albite content of plagioclase with pressure [26] . Plagioclase composition is pressure dependent, decompression causing more calcic plagioclase in equilibrium with liquid and sodic plagioclase dissolution [27] . A model of rapid magmatic decompression has been proposed for plagioclase resorption by Nelson and Montana [27] . The extent of resorption depends on the pressure drop and the re-equilibrium period after the depression.
Zoning of plagioclase is probably a function of cooling rate. Normal zoning in plagioclase could form at the indicated slow rates. With increased cooling rate, the core of plagioclase is more albitic [28,29]. Reverse zoned plagioclase can result from supercooling [30,31].
The plagioclase resorption texture in ECS can be the result of magma decompression. Bubbles in the resorbed plagioclase might result from a period of equilibrium degassing and bubble growth and then a rapid magma rise and eruption [32,33]. With rapid rise, the rim of plagioclase presents reversed zoning.
Hornblende usually occurs as jackets surrounding pyroxene and biotite in ECS. The relationship between hornblende and biotite reverses Bowen's discontinuous reaction series. However, biotites may crystallize before amphiboles for silicate melts near alkali feldspar saturation [34] . The H2O-conservative reaction
takes place between 200 and 4000 bars at 755°C with phlogopite-pyroxene- quartz as high-temperature assemblage [35] . For the reaction
the free energy is lower on the right hand side [35] . Only at the condition of low activities of SiO2 and KAlSi3O8 does Bowen's reaction series become the general case [35] . Decreasing thermal stability of amphibole with increasing pressure has been observed [36] . At similar temperatures but with decreasing pressure, the stability of amphibole will increase. Therefore, biotite coexist with pyroxene and biotite crystallized before amphibole has been demonstrated in many volcanic rocks [35,37-39].
4. Mineral Analysis
Electron microprobe analyses were performed for most phenocryst phases; representative probe analyses are listed in Table 2 (Supplementary File).
Mg/(Mg+Fe) for biotite and hornblende in ECS is similar (Figure 9a). They should have crystallized from the same source. For biotite with hornblende overgrowths and isolated biotite grains, the biotite with hornblende overgrowth has higher Mg/(Mg+Fe) (Figure 9b). Therefore, they are the products of earlier crystallization. Therefore, the overgrowth of hornblende around pyroxene and biotite in ECS is from a normal crystallizing sequence. The plagioclase, biotite, hornblende aggregates in the rock might have come from the cooling rim of the magma chamber and entangled during the magma ascent.
The Mg/(Mg+Fe) ratios in biotite and hornblende are totally different between SMR and ECS (Figure 9a), suggesting that SMR and ECS belong to different magmatic systems.
5. Geothermometry and Geobarometry
Several geothermometers and geobarometers were used to calculate the temperature and pressure for these units [40] (Table 3, Table 4) (Supplementary File).
Plagioclase for BB mainly falls in the field of andesine (An51-66). Alkali feldspar in BB is Na-sanidine. Plagioclase for BAT ranges from oligoclase, andesine, to labradorite. Most of plagioclase in BAT is compositionally zoned. For ECS, both Ba and Sr in plagioclase show positive partitioning with increasing whole rock SiO2 [40] . Alkali feldspar plots as K-high albite. Most plagioclases and the rim of zoned plagioclase for SMR are high-oligoclase (An13-17). Alkali feldspar of SMR is Na-sanidine (Or57-62). Sr and Ba partition into alkali feldspar in both ECS and SMR and show positive correlations with the Or content in the alkali feldspar [41] .
Using SOLVCALC 2 [42] , a two-feldspar geothermometer program, and the average composition of plagioclase rim and alkali feldspar, different geothermometers give similar results (Table 3) (Supplementary File), the temperature for BB is T=789°C [43] ; T=794 oC [44] ; and T=790°C [45] . The temperature for SMR is T=709- 711 oC [43] ; T=678-690°C [44] ; and T=708-720°C [45] . The SMR temperature is lower than reported data ~760°C for VGM [10] . Using the assumption that the cores of zoned plagioclase were in equilibrium with sanidine, the temperatures for crystallization of plagioclase cores ranged from 800-1000°C [46] .
Clinopyroxene and orthopyroxene coexist in the BB and BAT. Clinopyroxene for BB mainly falls in the augite field. Most clinopyroxene in BAT falls within the upper-left of augite field. The orthopyroxene in BB and BAT falls in hypersthene field (En50-77).
Using the QUILF95 program [47] and the composition of the average rim of cpx, opx, ilmenite and magnetite, the temperature of BAT is 864-868°C, log(fo2) is -10.67 to -10.98 and the temperature for BB is 834-853°C, log(fo2) is -10.95 to -11.11. Using the two-pyroxene geothermometer to evaluate the difference of temperatures for both clinopyroxene and orthopyroxene [48] , the rims of the pyroxene usually gave similar temperatures. Therefore, the temperature from the mineral rim is likely the temperature of magma at its eruption stage. For SMR, QUILF95 for two oxides gives 728-736°C, log(fo2) is -14.32 to -15.12.
Hammarstrom and Zen [49] , Hollister and others [50] proposed empirical correlations between the total Al content of hornblende and the estimated pressures of crystallization of calc-alkaline plutons. The barometer has also been experimentally calibrated using the assemblage of quartz + alkali feldspar + plagioclase + hornblende + biotite + Fe-Ti oxide + melt + fluid [51-53] . Anderson and Smith [54] calibrated the pressure using a revised expression incorporating the effect of temperatures.
Hammarstrom and Zen [49] , Hollister and others [50] proposed empirical correlations between the total Al content of hornblende and the estimated pressures of crystallization of calcalkaline plutons. The correlation is linear:
and
where P is pressure in kilobar, and AlT is total number of cations of Al per formula unit based on 23 oxygens. Using these two hornblende geobarometers and the composition of hornblende rim, the estimated pressure for SMR is 1.13-1.50 kbar, similar to the reported data 0.5- 1.5 kbar [10] . BAT is in the range 1.57 -- 3.64 kbar; BB is 1.96 -- 4.38 kbar. The barometer has been experimentally calibrated using the assemblage of quartz + alkali feldspar + plagioclase + hornblende + biotite + Fe-Ti oxide + melt + fluid [51-53] . Anderson and Smith [54] calibrated the pressure using a revised expression incorporating the effect of temperatures. The geobarometer from Anderson and Smith [54] yields an average pressure of 1.18-1.33 kbar for SMR, very close to the results from Hammarstrom and Zen [49] and Hollister and others [50] . The high numbers are from Schmidt [53] calculation. The Schmidt barometer was established under a higher experimental condition -- 2.5-13 kbar. The geobarometer from Anderson and Smith [54] is not suitable for the high temperature ECS, because the equation has a 1.3 to > 2 kbar derivation for the high temperature system. The geobarometer from Anderson and Smith [54] is not suitable for the high temperature ECS, because the equation has a 1.3 to > 2 kbar derivation for the high temperature system. We chose Hollister and others' [50] empirical Al-in-hornblende barometer as our pressure report: SMR 1.22-1.24 kbars, ECS 2.52-3.68 kbars. The calculated pressures from the different barometers are listed in Table 4 (Supplementary File).
The iron-titanium temperature and two feldspar temperature were the most stable geothermometers in this research. The Iron-titanium thermometer yielded the highest temperatures. The iron-titanium geothermometer and two-feldspar geothermometer have a deviation about 40-60°C. This is in the allowed range of error; therefore these temperatures are acceptable.
6. Hypothesis for the generation of ECS
The three members of the El Cajete Series: the El Cajete Pumice Fall (ECP), Battleship Rock Tuff (BAT), and Banco Bonito Lava Flow (BB) have similar mineral assemblages.
From ECP, BAT to BB, the silica increases by ~2 wt.%. Accordingly, other oxides also show relative changes. The trace elements show complimentary trends. ECP, BAT, and BB have different ranges on Rb- Sr, Zr, and Ba diagrams (Figure 6). For the El Cajete Series, the earliest eruptions (ECP) are less silicic overall and the composition range is widest; the last eruptions (BB) have higher silica. This character is opposite to the general eruption trend expected from a zoned magma chamber. A five-stage model of evolution and eruption for the ECS is presented below (Figure 10) to help explain this compositional inversion and other features of the ECS [55] .
At stage I, there is a deep subjacent magma chamber under the southwestern margin of the Valles Caldera. Through fractional crystallization, this magma chamber has developed vertical chemical zonation, with high silica rhyolite at the top overlying lower silica rhyolite. The crystallization temperature for BAT is 828-935°C (Fe-Ti oxides) and for BB is 835-925°C (Fe-Ti oxides). Ptotal is 3-3.6 kbars for BAT and 3.3-4.2 kbars for BB according to the inner rim composition of hornblende. The probable depth of the deeper chamber is 9-11 km.
Teleseismic 2-D surveys indicate a low-velocity zone exists in the midcrust beneath the Valles caldera [56-58] . Steck and others [59] reported a low velocity zone in the mid-crust underneath the northwest half of Valles caldera. The depth of low velocities is likely at 7.5 and 12.5 km (with top deeper than 5 km and shallower than 15 km) [59] . This may be a remnant of the deeper magma chamber proposed in our model.
In stage II, possibly induced by the intrusion of new magma below, the magma of this subjacent chamber is intruded up into a shallow-level chamber. During the intrusion, the vertical compositional zonation is converted into concentric zonation in the shallow chamber by passage through a narrow, connecting conduit. The intrusion led to some mixing between the adjacent composition zones and also caused lowering of pressure. The resorption of plagioclase, reversed composition zonation of plagioclase and the hornblende overgrowth may be the result of this process. This kind of intrusion has been documented in the Grizzly Peak cauldron, Sawatch Range, Colorado [60] . Because of the push from underneath, the most evolved chemical zones in the shallow chamber became thinner, whereas the same chemical zones on the sides of the chamber became thicker.
Stage III involves eruption of magma to form the ECP and the BAT members. Because of the strong degassing of the magma, these first eruptions are more violent than the later ones [61] . This explosion tapped deep into the shallow chamber; this deep tapping created mixing of the different chemical zones. Initial sub-plinian eruptions formed the largest eruption of the series, the El Cajete Pumice Fall. Then magma from the deeper levels and the sides of the chamber filled up the erupted space. This process mingled magma of different zones, causing the less evolved magma to fill the upper part of the chamber. The following eruption formed the BAT.
Stage IV. After the eruption of Battleship Rock ignimbrite, there was an eruption lull. During this static period, the magma chamber re-equilibrated and more evolved magma pooled in its upper part, reestablishing the normal compositional zonation. In this time, erosion removed some of the upper part of unwelded ignimbrite, thinned the BAT stratum and formed some gullies on the surface of BAT.
Stage V. The magma activated again. This time, a less energetic eruption only tapped the upper portion of the magma chamber. The issued lava formed the more evolved BB. Because the Banco Bonito lava is the uppermost and most evolved magma erupted from the chamber, it has higher silica and Rb, less Fe, Ca, Sr, and Ba.
7. Conclusions
The youngest eruptions in the Jemez Volcanic field occurred in the southwestern moat of the Valles Caldera. They formed two series: a younger El Cajete series (ECS), erupted at 48-61 Ka and an older South Mountain Rhyolite, erupted at 490-520 Ka.
ECS is rhyolite (SiO2 72.04-75.29 wt%); ECP has lower SiO2 (72.28-73.88 wt%), BB has higher SiO2 (73.08-75.94 wt%), BAT is intermediate. Phenocrysts for ECS are plagioclase, clinopyroxene and orthopyroxene, biotite, hornblende, ilmenite, magnetite and a few Nasanidine in the late stage rocks. The SMR is high-silica rhyolite (SiO2 76.3-79.25 wt%).
Using the iron-titanium oxides geothermometer basic program from Andersen and Lindsley [62] , the average temperature for SMR is 729-773°C, for BAT is 832 -- 840°C; and for BB temperature is in the range of 822 -- 863°C.
Two-feldspar geothermometry from different models produced similar results. BB is around 790°C. The temperature for SMR is about 700°C.
The iron-titanium temperature and two feldspar geothermometers were the most stable in this research. The iron-titanium thermometer yielded the highest temperatures. The iron-titanium geothermometer and two-feldspar geothermometer have a deviation about 50-60 oC. This is in the allowed range of error; therefore these temperatures are acceptable.
Pressures for ECS and SMR were calculated from different barometers (Table 4). The results for SMR are close, from 1.18 to 2.19 kbars. The results for ECS are in the range of 1.02 to 4.50 kbar, suggesting crystallization over an extended pressure/depth range.
Eruption dynamics caused the ECS to have a reverse compositional zonation. With increasing silica in ECP--BAT-- BB, Rb increases, and Nb slightly increases, whereas the other commonly incompatible elements, such as Sr, Ba, and Zr, decrease. U/Th dating of raw surfaces of zircon generated multiple ages from 75 to 350 Ka, indicating cycling of zircon crystals within the magma chamber [15] . A zoned magma chamber intrusion, mixing and decreasing eruption energy may have caused the inverted eruptive sequence for the El Cajete Series. The eruption of the ECS accompanied by progressive degassing of the magma chamber and declining energy of eruption. The initial plinian or suibplinian eruptions during strong degassing tapped into the deeper part of the magma chamber and caused mixing of different zones. During this eruption, the magma chamber released most of its volatiles. The last eruptions were less energetic. The youngest eruption tapped only the upper part of the magma chamber, which had re-established a normal vertical gradation during an eruption lull, and produced the BB lava, the most evolved part of the ECS.
Intrusion of new magma into the ECS chamber at depth may have triggered the eruption of ECS. Based on the geophysical research, the low-velocity midcrustal zone contains at least 10% melt and even may correspond to 62%-100% silicic melt [59] . Thus, a new pulse of magma into the midcrustal low velocity zone beneath western of caldera might cause a future eruption in the Valles region.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
We wish to thank Cleavy McKnight and the late Don Mullica of Baylor University for early review of this work, and the financial and moral support of the Department of Geology, Baylor University. We also thank the anonymous reviewer for their comments and corrections.