1. Introduction
Diabetes mellitus can result in multiple complications due to both microvascular and macrovascular physiological impacts, risk of hypoglycemic events and their aftermath, and mental health issues. All of these complications from poorly managed diabetes can increase the cost of care and lower the quality of life.
The treatment of diabetes mellitus is challenging for individuals living with diabetes, their healthcare professionals, and their family caregivers because successful management requires sustained self-management and lifestyle changes [1,2]. The challenges of self-management are first, providing an experience that enables people to manage their diabetes within their normal daily routine, and second, for these individuals to be aware of the right behaviors for successful diabetes management between healthcare professional visits. Even with remote patient management solutions, the experiences do not fully support self-management during routine daily tasks.
Digital health solutions can help people with diabetes self-manage, if they provide an experience that enables individuals to seamlessly use the solution throughout their day. The wide use of smartphones and mobile apps have shown promise for aiding self-management of diabetes mellitus [3], but they often have limitations that prevent long-term use. Mobile apps can often create more burden for people than benefit, which results in individuals discontinuing use of the app [3].
Mobile apps, many times, include sensor inputs that can also create limitations. Apps are often paired with sensors such as glucometers, scales, and blood pressure cuffs. This pairing enables simpler logging of data such as blood glucose and weight. However, these sensors can create limitations if they cannot be easily and routinely utilized on a long-term basis.
Most digital health solutions for diabetes perpetuate the traditional healthcare model of a live coach. The “digital health” is the connectivity between the coach and patient. These solutions have reported short-term results in 6-month duration, but little or no data is available for sustained use beyond 12 months [4]. In fact, a recent systematic review of remote patient management did not reveal a difference in HbA1c levels when using remote patient monitoring [5].
For mobile apps to have meaningful impact on people and optimize their healthcare behaviors, they must be capable of recognizing that all people are starting their self-management journey in different places. Not all people are ready to make changes in their healthcare and are at different stages of their ability to make changes [6]. Creating changes in healthcare behaviors can be done with multiple methods used over time by healthcare professionals, but these methods may not translate to use in mobile apps.
When a digital platform includes a simple experience, and the behavior change model is integrated into the digital solution, it may open the door to sustained improved results beyond the short-term results typically presented.
This clinical registry study follows commercial users of a complete digital health solution among people with Pre-diabetes, Type I, and Type II diabetes diagnosis. We hypothesized that during the first 6-months, improved control would be evident through improved glucose results. We additionally hypothesized that long-term control beyond 12-months would be improved due to the improved glucose testing experience and the addition of a digital virtual health assistant designed with proven behavior change methodology.
We present the following article in accordance with the TREND reporting checklist.
2. Methods
2.1 Platform [Technology design]
This study utilized the Pops digital therapeutic solution for diabetes self-management. The Pops solution combines an Artificial Intelligence (AI)-driven digital Virtual Health Assistant (VHA) named Mina, along with a glucometer that addresses the experience problems with traditional glucose test kits.
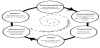
The VHA, Mina, is personified in the app. The app interface is intentionally designed with a consumer interface versus a traditional medical app. It is available for both the iOS and Android platforms. The VHA is also designed utilizing well-known behavior change science. First, the VHA assesses the user’s readiness to make healthcare behavior change per the Transtheoretical Model of Change. This model states that people fall in various categories of readiness such as preparing to change, acting on change, and maintenance, per Figure 1 [7]. The VHA continues to update which category the user is in based on the user’s interaction with the VHA.
The VHA then encourages change and movement to the maintenance stage by using another proven model, Motivational Interviewing (MI). MI is a proven technique to influence change in healthcare behaviors, especially in physical health conditions [8]. This method uses questions to help subjects realize themselves where they are compared to optimal behavior, which motivates them to change. All of the questions and interactions with the user are based on four principles as illustrated in Figure 2 [9]. It is key, in the use of MI, that no judgment is shown and that delivery of the MI technique is exhibiting the key principles.
The MI method was digitized within the Pops mobile app, and it is delivered by the VHA to the user to encourage change. The mobile app utilizes light coaching with the purpose of not causing users, who are managing a long-term chronic condition, to be annoyed by the VHA, which could cause them to delete the app. Light coaching results in a very calculated delivery of questions and information to the user, usually resulting in a digitized coaching opportunity approximately once every 48 hours and at a time the user is likely to be receptive. A typical coaching opportunity lasts less than five minutes.
The subject glucometer is designed to improve the experience of other glucometer test kits by eliminating the top three reasons people do not routinely use test kits during their normal daily routine [10,11].
Size: Users report that test kits which are large deter them from routinely carrying the test kit which is needed to test throughout the day. The Pops glucometer is 20% the size of a typical test kit, smaller than a modern mobile phone, and easy to carry around during a routine day.
Discreet: Traditional test kits require the user to assemble the kit which may not be convenient or discreet. The Pops glucometer requires no assembly to perform a test, making it simpler, faster, and more discreet.
Pain: Users complain of finger stick pain from the lancing tools in a test kit. This pain comes from the lancing tool, which requires loading a lancet into a spring within the tool. The Pops glucometer uses a design feature called stationary lancing, which enables it to be more stable and less painful [12].
To obtain a glucose result with Pops, the user slides open the meter cover, which turns on the glucometer and creates a Bluetooth connection with the app. The “check” button in the app on the phone is pressed, and one of the three indicators in the meter will illuminate to show which test to use. The user exposes only that test by pulling back a foil cover, uses the lancet to obtain a blood sample, and places that sample on the now exposed test strip window. Then the user replaces the foil cover and slides the meter cover closed.
The glucometer results are displayed on the phone and captured by the app. The VHA utilizes this data to provide new information to the user, the data is stored to allow the user to see trend information, and the data is captured in the cloud. Data can be shared with healthcare professionals and family or friend caregivers. Caregivers can set up alerts based on glucose results including hypoglycemic results.
Figure 3 illustrates a closed glucometer, Figure 4 shows the open glucometer with one test exposed, and Figure 5 shows an example question from Mina.
2.2 Measures
The primary outcome of this study is HbA1c movement during use of the Pops system. HbA1c is calculated using the following equation [13] from the actual glucose measurements that are recorded in the app through the user checking their glucose:
A1c = [2.52+[MBG/18.05]] / 1.583, where MBG is the Mean Blood Glucose of user-obtained glucose results. The Pops Rebel glucometer has previously been validated for accuracy in a clinical study [10].
All data was de-identified by assignment to a numerical ID for analysis. This results in safely handling the data and not having analysis biased by specific user characteristics.
2.3 Participants
The prospective study cohort is people who are part of a commercial clinical registry. The registry is an ongoing study of a growing number of users commercially using the Pops system. Active users are offered enrollment into the commercial registry up to a total of 500 users, and users self-select their inclusion into the registry. The study invitation strives to distribute participants into three categories: Pre-diabetes at ~20%, Type II diabetes at ~60%, and Type I diabetes at ~20%.
The study is currently limited to U.S. commercial users. They may be located anywhere in the U.S. with no other limiting factors that will exclude them. All study data for a user is compared to the same user’s baseline data using the Pops system. Being part of a commercial registry reduces the bias upon a participant when enrolled into a non-commercial study.
2.4 Analytical approach
All participants and their data are visible in the Pops database based on the wireless connection from the glucometer and Pops app to a cloud database. All users in the system are assigned a unique ID, which is the only identifier used in this study. All participants receive the same Pops intervention. 100% of the participants who enrolled remained in the study, and all of the users’ data was included in this study analysis. The first study participants began in February 2019. A download of the de-identified data was created to take a snapshot of the data as it existed for this study analysis on August 23, 2021.
All participants’ baseline HbA1c was calculated from the glucose measurements from their first use of the subject solution. HbA1c at 6-month intervals was determined through calculation of A1c with glucose measurements beginning at the 6-month interval.
Differences between the participants’ HbA1c results at baseline and subsequent periods of use of the subject platform were compared using a paired T-test with a significance level of 0.05, calculated using Excel. The hypothesis is that people with diabetes can sustain improved HbA1c results with a simpler self-management experience and digital virtual health assistant.
The study participants were first looked at as an overall homogeneous group. Sub-groups were then analyzed based on their baseline HbA1c level, the indicated type of diabetes, their sex, their age, and their frequency of blood glucose testing. Testing frequency may be a surrogate for engagement levels. No deviations from the protocol or analysis were made, and all data was included in each analysis.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki [as revised in 2013]. The study was approved by institutional clinical board of Pops Diabetes Care, Inc. and individual consent for this retrospective analysis was waived.
3. Results
3.1 Demographics
| Total cohort | 50 | |
| Gender | Male | 28 |
| Female | 22 | |
| Other | 0 | |
| Age | Mean | 54[SD = 13] |
| Minimum | 19 | |
| Maximum | 77 | |
| <31 years old | 2% | |
| 31 – 40 years old | 16% | |
| 41 – 50 years old | 16% | |
| 51 – 60 years old | 36% | |
| >60 years old | 30% | |
| Diabetes type | Type 1 | 18% |
| Type 2 | 52% | |
| Pre-Diabetes | 30% | |
3.2 HbA1c change analysis
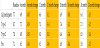
As illustrated in Table 1, the average HbA1c reduced in all timeframes observed for all types of diabetes and pre-diabetes. As one group, HbA1c improved significantly from 7.7% to 6.9% [p<0.001] after six months of use, HbA1c improved significantly from 7.7% to 6.6% [p<0.001] after 12 months of use, HbA1c improved significantly from 7.7% to 6.9% [p<0.001] after 18 months of use, and HbA1c improved significantly from 7.7% to 6.4% [p<0.001] after 24 months of use. Significantly the level of improvement sustained itself through 24 months of use of the Pops system.
3.3 Predictors of A1c change analysis
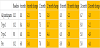
Additional analysis is performed considering other characteristics of the participants to see if one group has different outcomes or if a grouping can predict the improvement in HbA1c. In Table 2, the participants are broken down by their type of diabetes diagnosis. As illustrated in Table 2, every segment experienced a significant reduction in HbA1c at very similar levels with those participants with pre-diabetes experiencing the least improvement in HbA1c. Note that the participants with pre-diabetes also started with the lowest average HbA1c.
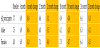
Table 3 shows the HbA1c change for those participants whose HbA1c started over 7.0%. A total of 21 participants had an HbA1c over 7.0% at the baseline. As one group, HbA1c improved significantly from 10.1% to 8.0% [p<0.001] after six months of use, HbA1c improved significantly from 10.1% to 7.3% [p<0.001] after 12 months of use, HbA1c improved significantly from 10.1% to 8.0% [p<0.001] after 18 months of use, and HbA1c improved significantly from 10.1% to 6.7% [p<0.001] after 24 months of use. Significantly the level of improvement sustained itself through 24 months of use of the Pops system.
Table 4 shows the HbA1c change by age group. Due to only an n= 1 in the <30 years old, this category is not statistically valid information but included for completeness. All other age groups showed statistically significant improvements in HbA1c over all time frames except for 31-40 year-olds in the first six months. However, that group does go on to show increasingly improved control over the next 18 months.
Table 5 shows the HbA1c change by sex. There are 28 males and 22 females in the study group. The female population started with a higher average HbA1c of 8.0% with males experiencing an HbA1c of 7.5%.
Table 6 shows the HbA1c change by glucose test frequency. Test frequency may be a surrogate measurement for how engaged a participant is in managing their diabetes. The people who are testing an average of 2-3 times per day were those seeing the largest improvement in HbA1c. This level of testing is a good indicator of user engagement. Even those less engaged at 0-1 tests per day saw smaller, but still improved, HbA1c. Those who were testing 4 or more times per day, saw no statistically significant improvement in HbA1c.
No adverse events of the approach were recorded.
4. Discussion and Conclusion
4.1 Discussion
The study analysis illustrates three important outcomes. First, people using the Pops solution are experiencing not only a significant improvement in HbA1c, but they are also sustaining the improvement after 24 months of continued use.
Often when technology is introduced for healthcare, only people who are already taking good care of their health will utilize the new technology yielding little additional benefit. The data in Table 3 above for those people who have a baseline HbA1c above 7.0% demonstrates the Pops solution can be a good solution for those people who have not been able to adequately manage their diabetes prior to use of the Pops solution.
Finally, engagement appears to be a good indicator of HbA1c improvement. Using tests-per-day as a surrogate metric of engagement, the data shows the greatest improvement in HbA1c when the participants are testing 2-3 times per day on average. It is interesting that the people testing more frequently show no statistical improvement, and it is theorized from the baseline HbA1c average of 6.3% that these people were previously engaged in self-management prior to Pops use. The Pops solution did not offer significant additional HbA1c reduction.
As this is a study built on an ongoing clinical registry, data will continue to be tracked past 24 months. Future studies of the registry data will include additional participants, improved compliance toward ADA guidelines, and glucose time in range.
4.2 Limitations
This is a clinical registry of commercial users. The study cohort is not visiting a clinical study investigator site. All the data and conclusions derived are based on what is visible from the Pops database and not on clinic in-person appraisal or lab testing.
4.3 Conclusions
Diabetes is a growing problem, and multiple current approaches to better self-management have not published sustained compliance and outcomes. Multiple solutions have shown promise with A1c improvement in the first six months of use, but there is little data for long term sustained use.
Pops is an approach to self-management that moves away from a focus on remotely managing patients. The Pops solution provides an experience that participants may be able to perform more easily for diabetes management while they perform other tasks in their lives.
These results demonstrate that an AI-assisted simpler experience for self-management improves sustained diabetes management results with a significant reduction in HbA1c at least out to 24 months of use.
Competing Interests
All authors have completed the ICMJE uniform disclosure form. TB is a non-paid advisor to, and a shareholder of, Pops Diabetes Care, Inc. LS is acurrent employee of Pops Diabetes Care.
Author Contributions
Conception and design: Tim Balder, Lonny Stormo
Administrative support: Lonny Stormo
Provision of study materials or patients: Lonny Stormo
Collection and assembly of data: Lonny Stormo
Data analysis and interpretation: Tim Balder, Lonny Stormo
Manuscript writing: Tim Balder, Lonny Stormo
Final approval of manuscript: Tim Balder, Lonny Stormo