Key Points
- First study to assess the prevalence of malignancy in predominantly Black RA patient population.
- The prevalence of malignancy was 11.2%, with breast cancer being the most frequent followed by colon and cervical cancer.
- No significant differences were found in patient comorbidities, smoking rates, clinical characteristics, medication patterns between the cancer and non-cancer groups.
1. Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by proliferating synovitis, cartilage damage and juxta-articular bone destruction that results in joint deformities and functional disability [1]. In addition, RA has also been associated with significant comorbidities, such as interstitial lung disease, cardiovascular disease, depression and an increased risk of both hematologic and solid malignancies [2,3].
While the etiology of RA is multifactorial with a complex interplay of environmental and genetic factors, the pathogenesis is rooted in chronic, systemic inflammatory activation and over activation of the adaptive and innate immune responses [1]. Similarly, inflammation is fundamental to the neoplastic process, whereby inflammatory cells are essential for tumor development, via increased cell proliferation, angiogenesis, evasion of the adaptive immune response, and facilitation of metastasis [4].
The direct link between RA and cancer dates to 1978, when Isomaki established that RA patients had a higher incidence of lymphomas, leukemias, Hodgkin’s disease, and myeloma [5]. Since then, numerous follow-up studies including single-center retrospective cohort studies as well as meta-analyses have corroborated Isomaki’s findings and expanded on his initial work, showing increased risk of lung cancer and lymphomas and a decreased incidence of colon and breast cancer among patients with RA [6].
Some of the increased risk of malignancy in patients with RA can be linked to common risk factors, with tobacco use lending a common predisposition to lung cancer and RA, and non-steroidal anti-inflammatory drug (NSAID) use, often employed by RA patients for pain control, conferring protection against the development of colon cancer. In the case of RA patients’ proclivity to development of lymphomas, it has been hypothesized that the continuous immunologic stimulation results in clonal selection and proliferation of B cells, which are the very culprits of certain lymphomas [6,7]. Given the clear link of immunologic stimulation to development of hematologic malignancies, there is a renewed interest in investigating the link between RA and malignancy.
In recent decades, the development of immunologic and targeted therapies has resulted in slowed disease progression and increased patient survival. While significantly improving disease burden and cause-related mortality, the advent of new therapeutic strategies including disease-modifying anti-rheumatic drugs (DMARDs) raises concern of their impact on concomitant malignancy rates in these patients, given the success of drugs rests on their mechanism of inducing immunosuppression. A recent review article suggested that use of biological DMARDs decrease the overall risk of developing malignancies with the exclusion of hematologic malignancies [8].
Importantly, most of the studies to date which highlight the incidence of malignancy and RA have been based on findings of a patient population wherein the majority are Caucasian, raising the question of the generalizability of these specific cancer type risks to other patient populations.
The goal of the present study is to describe the types of malignancies encountered in an urban, predominantly Black RA patient population.
2. Methods
In this cross-sectional, single-center study, we identified patients with RA as either principal or secondary diagnosis, via the International Classification of Diseases, Ninth Revision and Tenth Revision codes (ICD-9-CM 714.0 and ICD-10-CM M06.00–M06.09). All inpatient discharges of patients 18 years or older that took place at the State University of New York Downstate Medical Center- University Hospital of Brooklyn and New York City (NYC) Health + Hospitals/Kings County, from January 1, 2010 to May 30, 2017 were included. Our hospitals serve the predominantly Black population of Central Brooklyn, NY. The protocol was approved by the SUNY Downstate Institutional Review Board [1080808] and the Office of Research Administration at NYC Health + Hospitals/Kings County [001252].
We used physician entries (inpatient/outpatient notes and consultations) and the presence of disease-modifying anti-rheumatic drugs (DMARDs) in the medication reconciliation or DMARDs prescriptions.
Two investigators (IMM, SYZ) independently reviewed the cases identified by ICD codes to confirm RA diagnosis by the 2010 American College of Rheumatology criteria [9].
A standardized study collection data sheet was utilized for data abstraction for the confirmed cases; demographics, clinical data including history of smoking, year of RA-diagnosis, comorbidities, laboratory data, hand imaging and treatment regimens were collected. Cancer diagnosis was confirmed by two investigators (MSB, IMM).
A musculoskeletal radiologist (SK) evaluated the bilateral hand radiographs utilizing the Simple Erosion Narrowing Score (SENS) to ascertain the presence and number of erosions and joint space narrowing [10].
The radiologist was blinded to the serologic status of the cases reviewed. Data are presented as descriptive categorical variables or as mean ± standard error of the mean (±SEM), where applicable. We used t-test to compare between groups for continuous variables, and Chi square analysis for categorical ones. Data analyses were completed via IBM® SPSS version 23.
3. Results
Of the 1,142 RA patients identified by ICD codes, 281 had no clinical documentation to support RA diagnosis, 88 had insufficient data for confirmation of RA diagnosis, and 248 had an alternative diagnosis. Forty-eight records were identified as duplicates and abstracted as 24 unique patients. This process resulted in the identification of 501 patients with confirmed RA that were included in this analysis. Women represented 87.8% of the study cohort. Blacks accounted for 88.4% and Hispanics 9.2% of the patient population. Body mass index (BMI) was 28.9 ± 7.56 (±SD). Mean age was 65.2 ± 14.4 for women vs. 61.2 ± 17 for men (p < 0.04) (Figure 1).
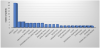
We encountered a cancer prevalence of 11.2% (56/501) in our RA population. There were 18 breast cancers (2 ductal carcinoma), four each for colon and cervical cancer, three each for lung, multiple myeloma, thyroid, squamous cell carcinoma and pancreas; two each for endometrial, Non-Hodgkin's lymphoma, meningioma and prostate and one each for urinary bladder, esophageal adenocarcinoma, lymphoma, glioblastoma, liver, Hodgkin's lymphoma, sarcoma, ovary and renal cell carcinoma. Four patients with usual interstitial pneumonia pattern of RA-related interstitial lung disease (RA-ILD) were found to have developed breast cancer.
In table 1, we present a comparison between the cohorts of RA with cancer and RA without cancer. We did not find significant differences between the cohorts in the clinical parameters such smoking history, body mass index (BMI), traditional cardiovascular (CV) risk factors, CV outcomes and extra-articular manifestations of RA. In regards to RA-specific characteristics, we found no differences in years of RA disease, joint erosion, joint space narrowing or SENS score except for significantly higher ESR among the cancer group and RF seropositivity in the non-cancer group. Therapeutic modalities for the two groups were also examined and exposure to corticosteroids, DMARDS and biologics revealed no significant differences.
We also assessed the sequence of cancer diagnosis to RA diagnosis for the 22 cases with available data. Twelve patients had developed cancer after RA diagnosis (mean, 10.75 years; range 5-22 years), 8 had developed cancer before RA diagnosis (mean, 6.75 years; range 3-11 years) and 2 patients received the two diagnosis the same year. Among the patients who had developed cancer after RA diagnosis, 66% had RA-specific risk factors however, no differences were found in regards to exposure to RA therapies prior to malignancy diagnosis.
The mean age at cancer diagnosis was 59.9±5.2 for the patients who developed cancer before RA diagnosis which was similar to that of the patients (58.25±16.02) who developed malignancy after RA diagnosis.
In regards to overall survival for the cancer cohort, 43.4% of the patients were alive, 32.1% of the patients were lost to follow-up and 24.5% had expired by the date of completion of the study.
A sub-analysis of the breast cancer patients showed that 11 were alive (61%), 3 had expired (16.6%) and 4 patients (22.2%) had been lost to follow up.
4. Discussion
The prevalence rate of cancer encountered in this predominantly Black RA population was 11.2%. The incidence of malignancy in RA in a recently published study from Beijing was 4.16%. Additionally, a prospective study conducted in Olmsted County, MN, showed a 23.8% incidence of malignancy among RA Caucasian patients [13,14].
The association of RA and malignancy was recognized in the late 70’s in an important study conducted on Caucasian RA patients with the number of malignancies such as leukemia, lymphomas, Hodgkin’s disease and myeloma were found to be more than double of the expected for the general population [5].
The association between RA and malignancies was again demonstrated in a meta-analysis based on published data on a Swedish RA population. The risk of lymphoma and lung cancer were noted to be higher for the RA patients while breast and colorectal cancers were lower among the RA cohort. Risk factors such as cigarette smoking, chronic lung inflammation, and presence of interstitial lung disease in RA patients were hypothesized to play a role in the development of cancer [15].
Published studies focusing on Caucasian RA population consistently reported the increased risk for lymphoma and lung cancer while cervical, prostate cancer and melanoma were seen with a frequency similar to that general population [16].
Another study on a Caucasian population reported a small to moderately increased risk of malignancies in the range of 5-10% among RA patients if non-melanoma skin cancers were excluded; the risk was highest for hematologic malignancies. Lung cancer risk appeared to be also increased. The risk of malignancy in RA did not appear to be related to the use of DMARDs or biologics such as TNF inhibitors [13].
When long standing and recent onset RA patients were compared in a large Caucasian cohort the risk of lymphoproliferative disease or solid tumor was not elevated [7,17,18].
Our population demonstrated a higher frequency of cancer than previously described among Caucasian cohorts and breast cancer was seen in 32.14% of the cases in contrast with 23% (17/74) found in the Beijing study. Conflicting reports exists in regards to breast cancer and its association with RA. One study reported breast cancer to be lower in RA patients than in the general population however, Blacks made up only 7% of the entire patient cohort [19]. On the other hand, increased risk of breast cancer in non-Caucasian RA patients was recognized in a meta-analysis [20].
The incidence trends of all site cancers and lymphoma, in Veteran RA population, showed a significant decrease with the introduction of biologics comparing 2001-2005 to 2006-2010. The population was predominantly Caucasian (80%) and might suffer from potential referral bias [21].
Our study demonstrated significantly higher ESR values among the RA group with cancer than without cancer (p<0.05). The association between inflammation and malignancy has been well-described, whereby persistent immunologic stimulation and the presence of inflammatory mediators leads to genetic instability and accumulation of random genetic alterations in cancer cells [13]. Further, high frequencies of p53 mutations similar to those found in tumor cells have been described in RA [15]. Additionally, immunostimulation, rather than immunosuppression mediated by RA treatments, has been considered the major driving force in the increased incidence of inflammation-associated lymphomas seen in RA [17,22].
The BMI between the two patient groups was not significantly different. However, there was a higher rate of obesity (BMI >30) among those with cancer consistent with the notion that obesity is associated with increased cancer risk.
Further, we observed significantly different rates in RF seropositivity between patients with and without cancer which likely express disease severity however, no difference was found in the frequency of DMARDs or biologics use between the groups.
The fact that we encountered a cancer prevalence of 11.2% among our RA population could be explained by the heightened surveillance provided to RA patients that allowed for early detection of malignancies given the use of DMARDs as therapeutic agents that are known to confer an increased risk of malignancy (Figure 2).
Finally, our study is limited by small sample size, the retrospective nature of the analysis, lack of RA-specific disease activity measurements, anatomic pathology characterization, and staging of the cancers, response to therapeutic interventions, changes made to RA therapy and survival outcomes. Inaccuracy in coding explains the number of cases in which RA diagnosis was not found in the clinical documentation. There is also data missing at random which we believe is harmless and does not represent a systematic bias.
5. Conclusions
This is the first study to evaluate malignancy in our predominantly Black RA population with a prevalence of 11.2%.
Breast cancer was the most prevalent malignancy among our Black RA population, which is consistent with previously published report that breast cancer risk is increased among patients with RA [20]. Cancer patients with RA were found to have a significantly higher ESR than non-cancer patients, likely reflecting higher levels of inflammation due to the dual disease process.
No significant differences were found in patient comorbidities, smoking rates, clinical characteristics, medication patterns between the cancer and non-cancer groups.
Further studies are needed to understand the higher overall risk of malignancy among our Black RA population. Possible risk factors include higher rates of obesity, smoking, advanced age, use of steroid and immunosuppressive drugs, as well as high levels of inflammatory markers among our patients.
Competing Interests
The authors declare that they have no competing interests. The author declare that there is no competing interests regarding the publication of this article.
Acknowledgments
We are grateful to Chao Ma, Sharlene Mills, Denton Smith, Stuart Clenman for their help with data retrieval and organization.