1. Introduction
Glomerulonephritis (GN) is a group of renal disease that injures glomeruli, with the first step of filtering blood to produce urine [1]. Many patients with glomerulonephritis have mild, asymptomatic illness that is not noticed by patients and thus, remained undiagnosed [2]. Unfortunately, most of the patients possess risks of kidney failure and premature cardiovascular disease [3]. It is the third most common reason that patients lose their kidney function in South Korea [4]. In U.S., about 23% of the patients with end-stage renal disease lost their renal function as a result of GN [5]. The socio-economic burden of GN is enormous in its contribution to kidney failure requiring dialysis. Moreover, medical expenditure for GN tends to be higher than the one for diabetic nephropathy since it occurs earlier in life and most patients with GN live longer compared to patients with diabetic nephropathy [6].
Treatment of GN usually depends on types of GN and the severity of illness. Although the etiology of this disease is not precisely known, immunosuppressants are commonly used because many types of GN are believed to be immune disorders. Tacrolimus and cyclosporine, called calcineurin inhibitors, are one of these immunosuppressants. According to clinical practice guideline for GN published by KDIGO (Kidney Disease Improving Global Outcomes), tacrolimus is recommended to treat several types of GN such as minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), idiopathic membranous nephropathy (IMN), and Lupus nephritis [7,8]. For all these indications, KDIGO recommends serum level of tacrolimus needs to be regularly monitored to avoid toxicity and maintain efficacy because of its narrow therapeutic window [8,9]. The problem is that maintaining tacrolimus serum level is tough. The pharmacokinetics of tacrolimus shows huge interindividual variability and various factors including drug interactions are all affecting tacrolimus pharmacokinetics [10]. That is why it has extensively studied in organ transplant population. Han NY, et al. demonstrated that hematocrit level, CYP3A5 genotype, and postoperative days were all influencing the clearance of tacrolimus. The authors also found body weight was associated with the volume of distribution (Vd) of tacrolimus in South Korean [11]. Population pharmacokinetics of tacrolimus in hematopoietic cell transplant (HCT) patients was also studied recently [12]. However, it was not studied in patients with GN patients.
Studying pharmacokinetics of tacrolimus in GN is important in that it is expected to be different from transplant population. The absorption of medications in chronic kidney disease (CKD) will be delayed due to decreased gastric emptying time and prolonged elimination half-life of the drug [13,14]. Distribution of the medication will also be affected by renal dysfunction. Many GN patients have low albumin level due to heavy proteinuria. As a result, hypoalbuminemia changes protein binding of drugs and increases the serum concentration of the free drug. Anemia is another factor that leads to pharmacokinetic changes. Since tacrolimus largely binds to erythrocytes, anemia of CKD may cause the alteration in tacrolimus pharmacokinetics [15]. Edematous status caused by proteinuria also alters the Vd of the drug [16,17].
There are a few studies regarding population pharmacokinetics of cyclosporine or mycophenolic acid in patients with GN [18,19]. However, none of them studied the pharmacokinetics of tacrolimus in GN. Due to huge inter-individual and intra-individual variability, predicting therapeutic effect of tacrolimus may be difficult. The association between tacrolimus pharmacokinetics and various factors (i.e. edematous status, hypoalbuminemia, and concomitantly used medication that can cause pharmacokinetic drug interaction with tacrolimus) were not clearly understood. Understanding of pharmacokinetics of tacrolimus is clinically significant due to its narrow therapeutic index and dose-related toxicities. Therefore, the objective of this study is to derive population pharmacokinetic model of tacrolimus in GN patients with identifyingto identify covariates that may be related to tacrolimus pharmacokinetics. It will improve pharmacotherapy with tacrolimus in patients with GN.
2. Methods and Materials
2.1 Study design and population
This is a population pharmacokinetic analysis study using the data from electronic medical records (EMR). We included all patients who diagnosed with GN and treated with tacrolimus-based immunosuppressive regimen at ambulatory clinic of Seoul National University Hospital outpatient clinics in South Korea between Jan 01, 2000 and Oct 30, 2013. All patients administered tacrolimus orally as hard capsule formulations twice daily. Patients under 18 years old and who were not reached the steady state condition of tacrolimus were excluded from the analysis.
2.2 Data collection and variables
EMR data were reviewed retrospectively to access clinical records, demographic data, and medication profile during study period. Clinical data included protein/creatinine ratio (PCR) in urine, complete blood counts including white blood cell (WBC), hemoglobin (HEMO), hematocrit (HCT), and platelet (PLT), renal function as blood urea nitrogen (BUN) andserum creatinine (SCR), cholesterol (CHOL), total protein (TPRO), albumin (ALBU), uric acid (URIC), total bilirubin (TBIL), aspartate transaminase (AST), and alanine aminotransferase (ALT). Demographic data included gender (SEX), body weight in kilogram (BWT), patient’s age (AGE), and types of GN by etiology (DZ).Generic names and dosages information of concomitantly used medications was collected about any known drugs that increase or decrease serum tacrolimus levels referenced by Micromedex®. Baseline characteristics and correlation analysis were performed by SPSS ver.19 (SPSS Inc., Chicago, Illinois, U.S.A.).All serum tacrolimus levels were trough concentrationsanalyzed by liquid chromatography-mass spectrometry using Quattro microTM API tandem mass spectrometer (Micromass, Manchester, UK). Before 2007, Microparticle enzyme immunoassay (IMX Tacrolimus II assay, Abbott Lab, IL, USA) was utilized for the analysis of tacrolimus levels.
2.3 Population pharmacokinetic analysis
The population pharmacokinetic analyses of tacrolimus were performed using non-linear mixed effect model (NONMEM®) software (version 7.2.0, ICON Development Solutions, Ellicott City, MD). Perl speaks NONMEM (version 3.5.3.), Xpose® (R package, version 4), and Pirana® (version 2.5.0) were additionally employed for model diagnostics and graphical presentation. The first-order conditional estimation algorithm with interaction (FOCE+INTER) was used throughout the analysis, considering interactions between pharmacokinetic parameters.
2.4 Structural model building
Both one-and two-compartment models with or without a lag time and first-order absorption and elimination were compared in this study [20-22]. Typical value of the absorption rate constant (Ka) could not be estimated because of the lack of concentration data in absorption and distribution phase. Therefore, Ka value was fixed at 4.5 h-1 which was previously reported in a study ofliver transplant patients [23]. Since the bioavailability of tacrolimus (F) could not be calculated in this analysis, tacrolimus clearance (CL) and apparent volume of distribution (V) were matched to CL/F and V/F, respectively. Interindividual variability of each parameter in structural model was estimated by an exponential error model using following equation:
Pi = θ × eηi
Where P stands for a pharmacokinetic parameter, Pi means the individual value for P in the ith individual, θ is the population mean value of P (i.e.,CL/F, V/F), and ηj implys an interindividual variability which is the difference between the observed and predicted value. Residual variability εij(ith individual, jth concentration), the difference between the observed (C(obs,ij))and model-predicted concentration (C(pred,ij)), was compared with additive, proportional, and exponential error model. Equations are as follows in order:
cobs, ij = cpred, ij + εij
Cobs, ij = Cpred, ij (1+εij)
Cobs, ij = Cpred, ij × Exp(εij)
Random effect parameters as η and ε were all presumed to be randomly distributed with a mean of 0 and variances of ω2 and σ2, respectively. Each different pharmacokinetic models were tested, and the best structural model was selected based on the goodnessof- fit after a visual predictive check (VPC), objective function value (OFV)of estimated models, and physiologic plausibility of parameter estimates.
Covariates were searched to evaluate the influence of demographics (SEX, BWT, AGE, DZ), clinical factors (PCR, WBC, HEMO, HCT, PLT, BUN, SCR, CHOL, TPRO, ALBU, URIC, TBIL, AST, ALT), and concomitantly used medications (i.e.,prednisolone, omeprazole, nifedipine, rifampin, fluconazole, rosuvastatin, omega-3-fatty acid). For continuous covariates, following equationwas used to test the significance of the covariates:
P=θ×e(θcovariate×(covariate-mean value of covariate))
The θ is population mean value of the pharmacokinetic parameter and θcovariate is the estimated effect of the covariate on parameters such as CL/F or V/F. For categorical variables (SEX, DZ, whether concurrent medications use), following equations were used for covariate modeling:
P=θ for reference covariate
P=θ×e(θcovariate) for exploratory covariate
The θcovariate is the assessed fractional change in θ for the exploratory covariate. Covariates were selected throughout a stepwise procedure by forward addition and backward elimination. Likelihood ratio tests were done by comparing differences in OFV between models. In forward addition step, covariates were selected if the reduction of OFV of 3.84 (χ2 distribution, P =0.05, degree of freedom=1) or more from base model was archived. During backward elimination, covariates at the P≤0.01 (in case of difference of OFV with 6.64 or more increase compared with previous model) were retained in the model.
2.5 Model evaluation and validation
The reliability and stability of the population pharmacokinetic model were tested by a nonparametric bootstrap procedure and VPC. During bootstrap procedure (n=1,000), data were randomly resampled to form a new data set from original ones. One thousand replicated data sets were simulated from each of the above-developed models. The observed data were covered with the 5th, 50th, and 95th percentiles of the simulated data calculated at each time point.
3. Results
One hundred and forty-five patients were included in this analysis and one thousand and nine hundreds thirty-eight blood samples were analyzed. The characteristics of included GN patients are summarized in Table 1 and 2. Patients were predominantly female (63.5%) and immunoglobulin A nephropathy was the most common diagnosis among included patients. The majority of patients seemed to have normal to moderately impaired renal function (estimated glomerular filtration rate: 76.58 ± 30.57 ml/min). Many patients also showed mild proteinuria and mild hypoalbuminemia. The average urine protein to creatinine ratio was 1.83 ± 3.0, and the mean serum albumin level was 3.82 ± 0.59 mg/dL. Concomitantly used medications that possibly have a pharmacokinetic drug interaction with tacrolimus were rosuvastatin(ROSU), steroids (STER), omeprazole (OMEP), nifedipine (NIFE), and rifampin (RIFA).
Although two compartments model with lag time was known to give the best fit for tacrolimus, two compartments model and absorption lag time model were tested and gave worse fit compared to one compartment model in this study. Hence, one compartment model with linear absorption and first-order elimination were selected and exponential error model best described the interindividual as well as residual variability. Among the tested covariates, PCR, WBC, HEMO, PLT, BUN, SCR, CHOL, TPRO, ALB, URIC, TBIL, ALT, BWT, and AGE were selected as covariates for the CL/F in the full model (ΔOFV >3.84; P< 0.05). Since hemoglobin (HEMO) highly correlated with hematocrit (HCT) as with AST and ALT, only the ones showed the larger decrease in OFV were selected. The inclusion of HCT, PLT, BUN, SCR, CHOL, TPRO, ALB, URIC, TBIL, AGE, OMAC, Steroid, OMEP, NIFE, RIFA, Dz in the covariate model for the V/F significantly improved the model (ΔOFV >3.84; P< 0.05). Again, hematocrit (HCT) was highly correlated with hemoglobin (HEMO) and thus, HCT was selected since it showed the larger ΔOFV.
During the backward elimination process to get the final model, all the covariates except serum creatinine in CL/F and V/F, and serum albumin in V/F were removed (ΔOFV> 7.88; P<0.005). The final model was as follows;
The predicted population parameter estimates obtained from the bootstrap procedure were highly correlated with the estimation of the final model. The estimated mean values were all within VPC estimates of 2.5th and 97.5th percentile range (Table 3).
4. Discussion
Although population pharmacokinetic studies were frequently performed in transplantation patients, this is the first study examined population pharmacokinetics of tacrolimus in GN patients and explored various factors including concomitantly used medications [24,25]. Other immunosuppressants such as cyclosporine and mycophenolic acid were studied in GN patients, but there is no information on tacrolimus pharmacokinetics in GN in the literature so far [19]. Understanding population pharmacokinetics of tacrolimus is critical in GN patients in that high serum level may decrease renal function even more in GN patients who are vulnerable to renal toxins. The Ministry of Food and Drug Safety (MFDS) in South Korea recommends checking the serum tacrolimus level regularly to avoid drug toxicity in lupus nephritis patients [7].In practice, poor correlation between dose and concentration was frequently observed due to pharmacokinetic characteristics of the drug. Because trial and error approach was commonly utilized in the management of GN patients on tacrolimus, the pharmacokinetic prediction would be helpful in deciding the tacrolimus dose and guiding the treatment regimen to treat GN. Hence, this study is designed to predict pharmacokinetics of tacrolimus in GN patients.
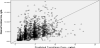
In this study, the estimated CL/F of 19.5 L/h was found (Figure 1 and Figure 2) to be in good correspondence with the previous reported value of 22.9 L/h although the V/F of 380 L was lower than the previously reported one [24,26-32]. This is explained by that a lot of previous studies were done in liver transplantation patients possibly with impaired hepatic function. Referenced by Prograf® package insert, theVd of tacrolimus was 0.85 L/kg in healthy volunteer, 1.07 L/Kg in renal impairment, and 3.1-3.9L/kg in patients with hepatic disease [33]. Therefore V/F of 380L, given Vd of 1.1875 L/Kg and bioavailability (F) of 0.2 in a person weighing 64 kg, seemed to be a reasonable estimate considering the renal and hepatic function of study subjects.
The final model reveals that serum creatinine level in CL/F and V/F, as well as serum albumin level in V/F, improved model prediction. Reduced renal function decreased the clearance of tacrolimus although tacrolimus is mainly excreted through hepatic pathway. In practice, patients with decreasing kidney function needs to have reduced tacrolimus doses and should be frequently monitored for serum tacrolimus level. Apparent tacrolimus volume of distribution was also reduced as deteriorating renal function. On the other hand, elevated serum albumin level causes plasma volume expansion and thus, increases the volume of distribution of tacrolimus in GN patients. Patients with hypoalbuminemia due to heavy proteinuria may require reduced tacrolimus dose and frequent monitoring of serum level. Many covariates were not selected in the final model. Protein creatinine ratio in urine was expected to be selected as covariates but the effect may be too small to show any significance in tacrolimus pharmacokinetics. Concomitantly used medications were also not selected in the final model. The effect of drug interaction might have been smaller than the one of random errors. Another possible explanation is that there were not many patients who were on medications that cause drug interaction. It led to reduced power to detect the difference.
There are a couple of limitations in this study. First, trough blood tacrolimus concentration was used in our analysis mainly due to data availability. Rich concentration data might precisely estimate pharmacokinetics of tacrolimus in GN. Second, although pharmacogenomicfactors are known to be strongly associated with pharmacokinetic variation of tacrolimus, it could not be tested in this study due to unavailability of data [24]. Consideration of genetic factors might have been improved the study result. Third, due to the retrospective study design, patients could not be controlled, and that leads to huge variation in serum tacrolimus levels. For instance, some patients took their blood level checked right after taking medications which was coincidentally found while reviewing patient’s records. Poor compliance in GN patients compared to well-educated transplanted patients was also observed. Transplant patients were provided with man to man medication counseling sessions by pharmacists at the practice site while GN patients were not. Additionally, many GN patients seemed to show poor understating of why they are taking immunosuppressant medications and why it is important to take medications correctly. Further study with prospective controlled design considering pharmacogenomics variables would give more predictive pharmacokinetic explanation in GN patients.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
MKS, NH and J-MO: Participated in research design.
MKS, JK, KC, NH and EJ: Participated in writing of the paper.
MKS, and MK: Performed data analysis.
J-MO: Made final revisions.