1. Introduction
From technological and pharmacological point of view, carbamazepine is an interesting API, being a poorly soluble model drug and the first-line drug for the treatment of epilepsy (including partial seizures, generalized tonic–clonic seizures, and mixed seizures) and trigeminal neuralgia. Preformulation studies are essential to estimate the ability of active ingredients or excipients to be processed through direct compression. In this sense, Suñé Negre et al. [1] at the University of Barcelona, Spain, have developed a new expert system called SeDeM, that supposes an interesting approach to the rational design of a formulation that will be adequate for direct compression [2].
The SeDeM method can be applied to active ingredients as well as to pharmaceutical excipients, providing information about their suitability for direct compression [1-3]. This system allows detecting the powder properties that need to be improved in order to facilitate the design of pharmaceutical formulations [4-7].
SeDeM is based on the experimental measurement of twelve rheological parameters, followed by a normalization of their values in order to build the SeDeM Diagram. This makes possible the comparison of the results of the different tests and provides the necessary information to decide whether a powder is suitable for direct compression as well as to identify the weak points that need to be corrected [8-9].
The considered parameters are the following:
- Bulk density (ρbulk)
- Tapped density (ρtapped)
- Inter-particle porosity (Ie)
- Carr index (IC%)
- Cohesion index (Icd)
- Hausner ratio (IH)
- Rest angle (α)
- Flowability (t’’)
- Loss on drying (%HR)
- Hygroscopicity (%H)
- Particle size (%Pf)
- Homogeneity index (Iθ)
These tests are grouped into five factors on the basis of the physical property of the powder that is being measured.
- Dimensional Parameter: Bulk density (ρbulk) and Tapped density (ρtapped). These affect the size of the tablet and its capacity to pile up. In addition, these tests are used in the calculation of other mathematical indexes for the determination of the compression parameter.
- Compressibility Parameter: Inter-particle porosity (Ie), Carr index (IC) and Cohesion index (Icd). These affect the compressibility of the powder.
- Flowability/Powder Flow Parameter: Hausner ratio (IH), rest angle (α) and flowability (t). These influence the flowability of the powdered substance when compressed.
- Lubricity/Stability Parameter: Loss on drying (%HR) and Hygroscopicity (%H). These affect the lubricity and future stability of the tablets.
- Lubricity/Dosage parameter: % Particles < 50 μm and Homogeneity Index. These influence the lubricity and dosage of the tablets.
Once these primary parameters are measured and normalised, secondary parameters such as Parametric profile (IPP) and Index of Good Compressibility (IGC)are calculated, to estimate the ability of a concrete powder to be processed by direct compression [9].
SeDeM Method allows a faster design of formulations, avoiding unnecessary studies and reducing the time of development, providing a guide for taking the appropriate ingredients to design a formulation [10], facilitating the robust design of the formulation from a science based perspective, in agreement with the principles of Quality by Design (QbD), described in the guidelines of the International Conference on Harmonisation (ICH Q8) [11].
The main aim of this study is to characterize API carbamazepine from the rheological point of view in order to know the ability of this drug to be processed through direct compression and to report the primary and secondary SeDeM parameters of carbamazepine, as well as its SeDeM diagram, contributing this way to building up a database that improves the formulation of Dosage Forms with this API.
2. Materials and Methods
Carbamazepine was purchased from Acofarma (batch 140143-N-1, Barcelona, Spain) and has been used for the present study.
Rheological studies have been carried out for the API following the new SeDeM Method [1,2]. Whenever possible, the methods indicated in pharmacopeias were applied. If not available, a system based on usual practice in Pharmaceutical Technology was employed, adapted specifically for the SeDeM Diagram [2].
Bulk density (ρbulk) and tapped density (ρtapped) were measured in accordance with the method described in European Pharmacopoeia, seventh edition [12].
Both parameters were determined in triplicate using 73.7 g of the carbamazepine into a 100 mL graduated cylinder readable to 1 mL.
The bulk density was obtained according to the Equation 1.
ρbulk=m⁄Vbulk
Where ρbulk is the bulk density (g/mL), m is the mass (g) and Vbulk is the apparent volume (mL).
The tapped density was determined from the tapped volume (Vtapped) occupied by the powder after repeated taps until the difference between successive measurements was lower than 2 mL. The volume taken was the value obtained after 1250 taps.
The tapped density was obtained according to Equation 2.
ρtapped=m⁄Vtapped
Where ρtapped is the tapped density (g/mL), m is mass (g) and ρtapped is the volume after tapping (mL).
Carr index (IC %) (Equation 3) and Hausner’s index (IH) (Equation 4) were calculated from the results of ρbulk and ρtapped and could be used to predict the compressibility and flowability of the powders. Sponginess index (IS) has been calculated according to equation 5.
IC%=(((ρapped-ρbulk))⁄(ρtapped)*100)
IH= ρtapped⁄ρbulk
IS=((ρtapped-ρbulk))⁄((ρtapped* ρbulk)
The flow ability was measured using the funnel described in European Pharmacopoeia [12]. This funnel has a height of 9.5 cm, an external diameter of 7.2 cm, an internal diameter of 1.8 cm and an angle of 45º with respect to the vertical.
The rest angle is an indirect method that indicates the cohesivity of a powder and is used to estimate its flow properties [13]. This is the angle of the cone formed when the product is dropped through a funnel placed at 2 cm of height with respect to the horizontal. This parameter is calculated according to the Equation 6.
tg(α)= h⁄r
Where r is the mean value of six measurements of the radius of the cone.
The loss on drying (%HR) is measured according to the method proposed by the European Pharmacopoeia. The samples were dried in a heater at 105ºC ± 2ºC until a constant weight is obtained. This assay has been carried out in three replicates.
The hygroscopicity was determined in three replicates as the increase in sample weight after being kept in a humidifier at relative humidity of 76 % (±2 %) and room temperature for 24 h.
The Percentage of particles below 45 μm has been determined as the percentage of particles that pass through a 45 μm sieve subjected to vibration for 10 min at speed 60 (Retsch, model AS 200, Germany).
The cohesion index was determined by compression of the powder in an eccentric tableting machine (Bonals A-300, Barcelona, Spain) using manual feeding and applying the maximum compression force accepted by the drug. The mean hardness (N) of the tablets is calculated.
For the determination of the homogeneity index, a sample of the API has been subjected to a sieving test employing the following sieve sizes: 0.710 mm, 0.500 mm, 0.355 mm, 0.180 mm, 0.090 mm, 0.045 mm. Sieves have been subjected to vibration during 10 min at speed 60 (Retsch, model AS 200, Germany). The percentage of product that is retained in each sieve has been calculated. Equation 7 is applied to the data obtained.
Where Iθ is the relative homogeneity index, informing about the homogeneity of the particle-size distribution in the range of the fractions under study. Fm is the percentage of particles in the majority range; Fm-1 is the percentage of particles in the range immediately below the majority range; Fm+1 is the percentage of particles in the range immediately above the majority range; n is the order number of the fraction studied under a series with respect to the majority fraction; dm is the mean diameter of the particles in the majority fraction; dm-1 is the mean diameter of the particles in the fraction of the range immediately below the majority range; dm+1 is the mean diameter of the particles in the fraction of the range immediately above the majority range.
Once the parameters described have been determined, they have been normalised, in order to situate their values in a scale from 0 to 10, in such a way that the maximum (10) and/or minimum (0) value of every normalised parameter corresponds to the natural limit of the parameter before normalisation. Table 1 shows the limits before normalisation [10] and the factor applied to each parameter.
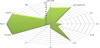
The normalised values, also called radius values r then plotted in the SeDeM Diagram (Figure 1). The figure drawn connecting the radius values with lineal segments depicts the characteristics of the powder for direct compression.
To determine whether the product is suitable for direct compression using a numerical method, the following indexes are calculated based on the SeDeM Diagram.
Parametric index (IP) has been calculated according to Equation 8.
IP= (No.ρ≥5)⁄(No.Pt)
Where No. p ≥ 5 indicates the number of parameters whose values are equal to or higher than 5.
No. Pt: Indicates the total number of parameters studied. The acceptability limit would correspond to:
IP ≥ 0.5
Parametric profile index (IPP) corresponds to the mean r value of all
parameters.
The acceptability limit would correspond to:
IPP= mean r ≥ 5
Good compression index (IGC) is calculated following equation 9.
IGC= IPP * f
Where f is a reliability factor and is calculated with the equation 10.
f=polygon area⁄circle area
The acceptability limit will be calculated by equation 11.
IGC= IPP * f ≥ 5
3. Results
As commented in the Introduction section, the SeDeM method provides information about the rheology of a powder, indicating its ability for direct compression [1,2]. Furthermore, this expert system shows the strong and weak aspects of the powder, providing a rational basis for the design of a direct compression formulation, correcting the weak points of a substance with other components of the formulation.
For this purpose, the rheological parameters were obtained in accordance with the described methodology. After that, the Diagram radii were calculated applying the equations shown in Table 1. The radius for Loss on drying was calculated as indicated in Table 2, according to previous papers in the field [1,2]. Table 3 shows the results obtained for the characterization of carbamazepine.
The results were graphically expressed in form of a SeDeM diagram (Figure 2). This provides intuitive and valuable information about the type of substances that would be adequate for obtaining a direct compression powder blend containing the active pharmaceutical ingredient carbamazepine. The results show that it is possible and feasible to obtain tablets of carbamazepine by direct compression with the addition of adequate fillers (Direct Compression fillers).
4. Conclusion
The SeDeM Expert System provides reliable and reproducible results for the technological characterization of powdered substances with respect to their suitability for direct compression.
Carbamazepine tested in this study has an appropriate ability to be used for direct compression. Using the SeDeM diagram, it can be easily appreciated that carbamazepine shows as strong points it’s very low relative humidity and hygroscopicity and adequate densities. The weakest point in the rheological properties of carbamazepine is the flow ability. Therefore, this API will notably benefit from the use of Flow Aid excipients and Direct Compression fillers.
Competing Interests
The authors declare that they have no competing interests.