1. Introduction
Cerebral revascularization procedure, especially external carotidinternal carotid (EC-IC) bypass surgery, has been used for the treatment of various cerebrovascular diseases, such as giant aneurysms, moyamoya diseases, and cerebrovascular occlusion refractory to medical treatment [1]. Whatever the anticipated indications, one of the main issues is to avoid bypass graft failure even after a successful anastomosis procedure.
Early detection and correction of possible factors that contribute to graft failure is important peri-operatively. Among all possible contributing factors, donor artery problems can occur in nearly 15% of bypass procedures [2]. Depending on the timing of occurrence, arterial flow passing through the donor artery can be compromised by various mechanisms, such as vasospasm, intimal hyperplasia, and etc [2,3]. Therefore, early detection of the donor artery compromise is mandatory, and early rescue of the donor vessel has been reported to be effective and safe for bypass graft saving.
With the advantages of hybrid operating suite, we are nowadays able to perform both neurovascular surgeries and neuroendovascular procedures at the same intraoperative period. Herein, we demonstrated how bypass graft patency could be evaluated during the EC-IC bypass surgery, and how we could detect the donor artery problem and rescue the artery immediately by neuroendovascular procedures during the operation.
2. Case Presentation
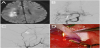
A 51 year-old-male, with past histories of diabetes, hypertension, and hyperlipidemia, presented to our institution with left-sided weakness and crescendo transient ischemic attacks at the right hemisphere despite of aggressive medical therapy. Magnetic resonance imaging (MRI) of the brain revealed an acute internal border zone infarct in the right centrum semiovale on diffusion-weighted imaging (DWI), indicating hemodynamic insufficiency at the right hemisphere (Figure 1A). Catheter angiography showed a high grade stenosis of the first segment of the right middle cerebral artery (MCA) (Figure 1B). The stenosis was angiographically characterized by a network of fine but tortuous arterial twigs, and its distal territory was perfused in a much delayed fashion. As both clinical and radiological features fulfilled the criteria of medically refractory MCA stenosis, the patient was planned for cerebral revascularization procedure using superficial temporal artery (STA) to MCA (cortical branch) anastomosis.
The whole procedure was performed in the hybrid operative suite equipped with an ArtisZeego FD system (Siemens AG, Forchheim, Germany) mounted on a robotic C-arm, which enabled us to perform both cranial surgery and intraoperative neuroendovascular procedures.
The whole procedure was performed in the hybrid operative suite equipped with an ArtisZeego FD system (Siemens AG, Forchheim, Germany) mounted on a robotic C-arm, which enabled us to perform both cranial surgery and intraoperative neuroendovascular procedures.
With the head in a radiolucent skull clamp turned to the left side, both frontal branch of the STA and the main STA pedicle were dissected from the scalp first (Figure 1C). Following temporalis muscle division and fronto-temporal craniotomy, the dura was opened in a cruciate fashion and a cortical branch of MCA suitable as the recipient vessel was identified. A desired length of STA was then decided and the STA was cut in a fish mouth fashion. The lumen of the STA was cleaned with heparinized saline. Stripping of the distal STA adventitia was also performed to remove the sympathetic endings on the vessel wall. The last step to prep the STA pedicle was to expand the vessel using pressure distention technique. Finally, a standard STA-MCA end-to-side anastomosis using 10-0 nylon sutures was performed (Figure 1D). During the anastomotic procedure, the main STA pedicle was temporally clipped, and was covered with a cotton gauze soaked with nimodipine.
Following completion of the anastomosis procedure, we covered the craniotomy wound with a sterile dressing for the following angiographic procedure. We placed a 5 Fr femoral sheath in the right femoral artery, and advanced a 5 Fr H1 diagnostic catheter to the right external carotid artery. The intraoperative angiogram confirmed that the anastomosis was patent, but the angiographic vasospasm of the STA pedicle was evident, which resulted in significant reduction of the flow across the anastomosis (Figure 2A). In order to relieve the vasospasm, we navigated a 2.5 Fr Cantata microcatheter (Cook Inc., Bloomington, Indiana), in the aid of a Transcend 0.014-inch microwire (Stryker Inc., Kalamazoo, Michigan), to the proximal segment of the STA pedicle (Figure 2B). 3 mg of nimodipine was then infused slowly under close monitoring of blood pressure. After infusion, the vasospasm was relieved, and the follow-up angiography demonstrated marked improvement of the flow through the bypass graft (Figure 2C). The dura was then closed loosely, and the bone flap was placed back. The temporalis muscle was approximated not to compress the STA. The wound was closed with nylon stitches. There were no complications throughout the procedures.Follow-up computed tomography angiography (CTA) at postop 1 month showed persistent patency of the graft (Figure 2D). The patient also had no stroke attacks in 6-month follow-up.
3. Discussion
The patency rate of a STA-MCA bypass graft could be as high as 96% at the long-term follow-up [4]. However, to maintain the high patency rate, various surgery and graft-related factors should be considered and optimized [1,2].
STA-related factors that affect STA-MCA graft patency included the length of the STA pedicle and the method to prepare the donor vessels. The STA length should be optimized to allow a tensionless suture with recipient vessel as well as to avoid graft bending or compression by the bony graft and temporalis muscle. Another three preparation steps beneficial for vasospasm prevention has also been reported. First of all, intra-luminal blood should be cleared with heparinized saline, but, during irrigation, only small and blunt-tip needle could be introduced into the STA lumen to avoid intimal injury. Second, pressure distension technique, a method to expand the vessel, was also effective to reduce postoperative graft vasospasm. [5] Third, sympathetic nerve endings observed in STA adventitia were also highly related to the development of the graft vasospasm [6]. Stripping of STA adventitia has been shown to improve hemodynamic function of STA,[7] but the extent of the stripping should be limited to the distal segment of the STA in order to preserve the vasovasorum which supplied the STA pedicle.
With all the techniques mentioned above, the flow within the donor vessel may still becom promised in the different time frames following the anastomosis. During the ultra-early and early stages, donor vessel might become narrowed secondary to the development of vasospasm, and one of the explanations for this phenomenon is the overt arterial stimulation exerted by the temporal clip application during anastomosis [2]. In contrast, stenosis of the donor vessel during the medium to late follow-up period is generally attributed to the development of intimal hyperplasia or thrombosis within the arterial pedicle [2]. Based on these mechanisms, neuroendovasculartherapy for hemodynamically relevant donor artery stenosis, by using either intra-arterial infusion of vasodilator or balloon angioplasty of the proximal arterial segment, have been advocated to be beneficial and safe [2,8].
As shown in our case, though anastomosis procedure was successful, the STA pedicle became so narrowed that that it might cause future bypass graft failure if the STA problem was not detected and solved intraoperatively. Since STA narrowing was observed immediately after the anastomosis procedure, we believed that STA vasospasm was the underlying mechanism, which was presumably attributed to previous temporal clip application on the STA pedicle. Since our angiographic equipment was already on board, therefore, the quickest way was to infuse vasodilators intra-arterially inside the STA pedicle. This maneuver successfully relieved the vasospasm observed in our case, and the graft flow significantly improved. If intra-arterial vasodilator infusion failed to relieve the vasospasm, or the vasospasm occurred again in the later period, balloon angioplasty of the STA pedicle might be our next treatment of choice to rescue the donor artery.
Some technical tips of neuroendovacular therapy should be emphasized to avoid possible complications. First, microwires and microcatheters should never be accessed across the anastomosis site in order not to cause graft disruption. Second, catheter-induced vasospasm or dissection of the donor artery was not uncommon, and it may aggravate the pre-existing donor artery problems [2,8]. To avoid this complication, especially when intra-arterial vasodilator infusion was planned only, neuro-interventionist should place the microcatheter in the target vessel as proximally as possible. Some authors also advocated just to use the diagnostic catheter for vasodilator infusions, eliminating the need to position the microcatheter into the donor artery [8].
4. Conclusion
We demonstrated that, during EC-IC bypass surgery, intraoperative confirmation of graft patency and immediate rescue therapy of the donor artery problem were technically feasible. This hybrid approach, as demonstrated by our case, was able to detect graft problems and avoid future graft failure in an earlier fashion. We believe that this will be an excellent example showing the advantages of concomitant neurosurgical and neuroendovascular approaches as mutual back-ups in the modern era of treatment strategies for cerebrovascular diseases.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Szu-Kai Hsu: Manuscript writing
I-Chang Su: Conception and design, Manuscript writing and
editing