1. Case Representation
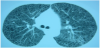
We present the case of a patient whose diagnostic suspicion is a lymphangioleiomyomatosis, and, for a definitive diagnostic, retroperitoneal adenopathies biopsies are needed. A 43 year-old woman, smoker of 4 cigarettes/day, with clinical records of extrinsic bronchial asthma, common migraine, iatrogenic hypothyroidism due to a total thyroidectomy for multinodular goiter. She is in medical treatment with levothyroxine 175mg/24h, budesonide/formoterol 160/4.5mcg, every 12 hours, and Montelukast 10mg/24h. The patient reported progressive dyspnea during last two months, becoming on minimal exertion and followed by occasional hemoptoic sputum. The physical examinations reveals mild bilateral crackles, and among the diagnostic tests, in the chestradiography bilateral reticulonodular interstitial infiltrates are found with predominance in the inferior lobes, and the thoracoabdominal CT scan reveals the bilateral presence of multiple cysticlesions in lungparenchyma, specially in the inferior lobes. Mucus or pseudonodular condensation fills some of those cavities and also multiple retroperitoneal adenopathies with pathological size are found (figure 1). The spirometry shows moderate obstructive pattern with positive response to bronchodilator, mild volume loss and a moderately reduced corrected diffusing capacity. Arterial blood gas analysis: pH=7.44 pO2=51.9mmHg, pCO2=40.3mmHg, Bicarbonate=26.6mmol/L, SaO2=88%.
Due to clinical manifestations, and the tests, biopsy sampling is needed for further diagnostics: given the severe lung affection and the high risk of iatrogenial complications related to the pulmonary biopsy sampling, despite the high diagnostic rentability, the sampling is decided to be retroperitoneal, specially in the right iliac lymph node chain, on account of its size.
At the operating room reception, the patient is found in good condition, eupneic, and without any new clinical manifestations. When monitored, we found SaO2=91%, heart rate= 73 bp and non invasive blood pressure=130/79. Given surgery, with a subumbilical midline incision, the planned anesthetic management includes a neuraxial regional block in order to avoid patient intubation, high positive airway pressures and the high risk of pneumothorax. A lumbar epidural catheter is finally decided for intraoperative and postoperative anesthesia. Complementary, a 16G peripheral venous access is placed, as well as nasal cannula at 3lpm, premedication with 2mg of midazolam, 50mcg of fentanyl, and ranitidine 150mg, 100mg of hydrocortisone for preventing adrenal insufficiency, and Ringer Lactate 500ml.
With a medial epidural approach at L1-L2, 17G 80 mm Tuohy needle, the epidural space is found at 6 cm from the skin, administering 3 ml of bupivacaine 0.25% with adrenaline at 1: 200.000, and the catheter is placed at 9 cm from skin. After 6 minutes from the administration of 10 ml of 0.5% isobaric bupivacaine and 50 mcg of fentanyl, the patient presented thermal dissociation, complete anesthesia, and weakness of both lower limbs. Before the surgery, another bolus of 5ml of 0,5% isobaric bupivacaine was administered. Besides, we combined the regional anesthesia with a conscious sedation with a continuous infusion of propofol at 2mg•Kg-1•min-1, maintaining a Ramsay Scale Level of activity of 4.
During the whole surgery, that lasted 1 hour, the patient remained hemodynamically stable, with SpO2 between 91% and 96%, with a maximum blood pressure of 135/91mmHg and minimum of 98/57mmHg, and with an suitable perioperative pain control, and arrives the reanimation unit without incidence. Three hours later, the patient was discharged with a complete recuperation of the motor block, with a Bromage Scale=1.
2. Discussion
Lymphangioleiomyomatosis is a poorly common disease, which only affects women, and in most cases, in their childbearing age. Histopathologically, an typic proliferation of smooth muscle around vascular and lymphatic structures, and around pulmonary interstitium is found, which tipically are pleomorphic and positive to HMB-45 monoclonal antibody. As well as the typical affection of the terminal airway with cystic-like diffuse dilatations we can also find hilar, mediastinic, and retroperitoneal lymph nodes. Although pathogenesis is unknown, it seems to be related to the loss of tumorsuppressor enzymes sharing pathogenesis with tuberoussclerosis, even though they are considered two different diseases [1]; yet it is clear the influence of the estrogens in the progression of the disease.
The average age of patients affected is 44 years old being 41 years the mean age of presentation. The most common clinical manifestations are progressive dyspnea, with even 2 years of slow development (70%), spontaneous pneumothorax (the debut symptom in 37% of cases, and it appears in 50% of the patients during the development of the disease), hemoptysis, and productive cough. Other symptoms that can be found are chylothorax, chylous ascites, lymphangioleiomyomas, kidney angiomyolipomas, that can be found in almost 50% of patients [2]. Pulmonary function tests reveal obstructive or mixed pattern. Frequently, the lungs appear to be hyperinflated with an increase of total pulmonary capacity and residual volume. It can also be found a reduced diffusing capacity, loss of pulmonary compliance and increased airway resistances that justifies the clinical manifestations of the patients.
The high resolution CT scan provides the suspected diagnosis and it has prognostic value [3]. Sometimes, in fact, typical clinical manifestations in a middled age woman, with emphysematous pattern, recurrent pneumothorax and chylous pleural effusion, the TC scan can provide the definitive diagnosis without the need of biopsy. However, the pulmonary biopsy is the test to establish the firm diagnosis [4].
The average survival rate is from 8 to 10 years, although it depends of the debut manifestation, and the severity of the pulmonary affection. An effective treatment haven't been found yet, although some authors recommend hormonal suppression in premenopausal women [5]; other therapeutical options are sirolimus and lung transplantation [6].
3. Conclusion
Given the severe lung affection of our patient, and the high risk of complications if connected to mechanical ventilator, such us hemoptysis or pneumothorax, described in literature [7] we decided a regional anesthesia technique with perioperative epidural anesthesia combined to conscious sedation, giving the patient a secure and confortable anesthesia during the whole surgical procedure and the immediate postoperative.
Competing Interests
The authors declare that they have no competing interests.