1. Introduction
Sinus of Valsalva aneurysm (SVA) is a rare cardiac anomaly, and it is typically of congenital origin [1]. SVA usually enlarges over time and remains silent until it ruptures. Because rupture of SVA is usually fatal, prompt diagnosis followed by surgical correction is mandatory [1-3]. Two-dimensional echocardiography plays a main role in the diagnosis of SVA rupture. However, entirely depending on echocardiography sometimes leads to misdiagnosis, and it can be dangerous, especially in the case of poor visualization or when combined with other structural abnormalities. In such cases, it has been reported that computed tomography (CT) can provide excellent diagnostic value for SVA rupture and combined anomalies with better spatial resolution of cardiac structure [4-6]. Herein, we present a typical case of ruptured SVA that was suggested by transthoracic echocardiography and confirmed by cardiac CT.
2. Case Report
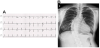
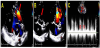
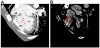
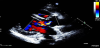
A 34-year-old woman visited our outpatient clinic due to dyspnea and chest discomfort newly developed one month ago. The symptoms progressively worsened during a several months’ period. Dyspnea was aggravated by exertion. Substernal chest pain was intermittent and of squeezing nature and it was not related to exercise. She had been heard about a hole between cardiac chambers when she was young; however, she did not remember the correct diagnosis. She had no other significant prior medical history except scoliosis. She was not a smoker and had no family history of heart disease. On initial examination, her general condition was good with blood pressure of 120/50 mmHg, and pulse rate of 77 per minute. She was afebrile, and there was no jugular venous distension or peripheral edema. There was a loud continuous murmur along with the left parasternal border line accompanied by a thrill. There were no other remarkable findings in chest and heart examinations. Electrocardiogram (ECG) showed nonspecific ST-T segment changes with normal axis (Figure 1A). Chest X-ray showed mild cardiomegaly and curved thoracic vertebrae (Figure 1B). There were no specific findings in blood tests including cardiac enzyme, inflammatory markers, rapid plasma regain test for syphilis and human immunodeficiency virus test. Transthoracic echocardiography (TTE) revealed normal size and function of both left and right ventricles (LV and RV). Color flow and pulsed wave Doppler identified a jet flow from the aorta to RV, at the level of the aortic root, seen during both systole and diastole (Figure 2 and Videos 1 and 2). There was no valvular stenosis or regurgitation of the aortic valve (AV). Rupture of SVA was suspected. However, the aneurysmal sac was not clearly defined by TTE, and other cause of intra-cardiac shunting, such as ventricular septal defect (VSD), could not be ruled out. Therefore, ECG-gated computed tomographic (CT) coronary angiography was performed to obtain information on additional cardiac pathology and relation to the coronary arteries. CT scan revealed a ring-like structure originating from the right coronary sinus of AV protruding to the RV. The ostium of the right coronary artery was not involved by the aneurysm, and there was no significant stenosis of coronary arteries (Figure 3A). Extravasation of the radiocontrast agent from the SVA to the RV was also seen on CT scan (Figure 3B). Based on these findings, the patient was scheduled for surgery. Immediately before surgery, transesophageal echocardiography (TEE) was carried out for surgical planning. The findings of TEE were concordant with those of aforementioned cardiac CT. TEE revealed a protruding aneurysmal sac with shunting from the right coronary sinus to RV (Figure 4 and Video 3). Invasive cardiac catheterization was not performed. Surgical findings confirmed a large aneurysm with a defect at right coronary sinus of the AV. There was no other combined cardiac pathology. Resection of the aneurysmal sac and repair of the aortic root using an autologous pericardial patch was performed. After surgery, TTE no longer showed abnormal shunt flow at the aortic root except minimal valvular regurgitation of the AV (Figure 5 and Video 4). She has been doing well without chest symptoms after surgery.
3. Discussion
Early recognition of ruptured SVA is very important because rapid surgical correction is lifesaving. In most cases, ruptured SVA can be correctly detected using echocardiography. However, TTE can miss diagnosis in the case of poor image quality or poor visualization. Although image quality is better, TEE cannot be applicable to all patients. In spite of good echocardiographic window, in some cases, a diagnosis of SVA rupture is difficult to establish with echocardiography even with cardiac catheterization [4,7]. Since SVA rupture is frequently misdiagnosed as other cardiac disease, clinicians can miss the opportunity to cure such patients. This situation is usually associated with fatal outcomes [7]. The most common cardiac pathology confused with ruptured SVA is VSD with or without aortic regurgitation. It has been demonstrated that supracristal VSD just below the right aortic cusp is associated with significant aortic regurgitation in approximately half cases, and thus can mimic ruptured SVA [8]. Unfortunately, many cases of VSD are combined with SVA rupture, especially in the Asian population [8-10]. A diagnosis of ruptured SVA becomes more difficult in the coexistence of VSD and ruptured SVA, particularly in the presence of significant aortic regurgitation. Besides VSD, patent ductus arteriosus, atrial septal defect, AV defect, tricuspid regurgitation, coarctation of aorta, or coronary or pulmonary arteriovenous malformation have been shown to be associated with SVA [10,11]. In order to rule out these complex conditions, additional information from other imaging modalities, such as cardiac CT or cardiac magnetic resonance imaging (CMR) may be useful [4-6,10,12, 13]. Cardiac CT can provide precise information on cardiac structure without disturbance by the lung and other artifacts. Coronary anatomy is also well visualized by CT [14]. With technical advancement, nowadays, the quality of CT is significantly improved, and thus, invasive cardiac catheterization is not routinely required for the diagnosis of ruptured SVA. In addition, information on involvement of the coronary artery or coexistence of significant coronary stenosis is sometimes essential for the consideration of concomitant coronary bypass surgery, especially in older patients with risk factors [12,15]. Some authors have advocated that routine coronary angiography should be performed to determine combined coronary anomalies in patients with SVA [16]. Another strength of CT is that it can detect combined aortic pathological conditions such as aortitis, aneurysmal dilatation or dissection, associated with SVA [6]. In our case, although VSA rupture was suspected by TTE, the presence of VSD was not ruled out because she had told us a hole between her cardiac chambers. Also, the typical morphology of aneurysmal sac was not clearly visualized on TTE. For these reasons, further tests confirming SVA rupture and other combined anomalies were needed. Cardiac CT showed a clearly defined aneurysmal sac and extravasation of contrast medium confirming the ruptured SVA. Cardiac CT also revealed a normal coronary artery with no other structural abnormalities. These findings are compatible to those of TEE and operation.
4. Conclusion
In conclusion, we report a case of ruptured SVA in a young woman. Diagnosis was suspected by TTE and confirmed by cardiac CT. The patient was stable after surgical correction. CT scanning may be useful in for diagnosis of ruptured SVA by providing more detailed visualization of adjacent structures and additional information on combined cardiac abnormalities.
Competing Interests
The authors declare that they have no competing interests.