1. Introduction
Walking is common and essential movement in various daily and physical activities.In the walking of human, it was happened command in the upper central, such as the cerebral cortex, and activating the brain stem, finally it is transmitted to the central pattern generator (CPG) of the spinal cord to the muscle of the effector activities during began to move with the objective. However, it is occurred to the walking exercise by the muscle activation, even if the no active control of upper central [1].
The study of the elderly people suggested that the walking speed and swing time variability were significantly decreased during 3s and/ or 7s of the dual task walking compared to normal walking [2]. The walking speed and swing time variability of walking of fallers were significantly lower than that of non-fallers [3]. In the previous study of compared to older adults with mild cognitive impairment (MCI) and normal control subjects, the walking speed, time of stride, and gait variability were significantly decreased by MCI group compared to control group. In addition, change rate of walking speed of MCI groups were significantly decreased in dual task conditions [4]. In the previous research investigating relationship between the cognitive function and EMG activity of muscle, coactivation time of EMG activities of agonist and antagonist during walking was significantly increased in the elderly people [5].
From these previous researches, it was suggested that the increases of walking speed and swing time variability during dual task walking in people of decreased cognitive function is closely related to the risk of the fall. The increase of coactivation of agonist and antagonist muscle might be a phenomenon to support the fragile joint of elderly peoples. It was not observed increased of the coactivation time by elderly people compared with young adults, because they have adequate of lower muscle strength [5]. The measurements of cognitive function during dual task condition were thought to be needed.
The concurrence of locomotion and another motion, termed dualtask walking, has received a lot of attention in some researches [6-10].
The adult human walk pattern was affected by ageing. Previous researches suggested that the walking speed and stride length decrease, while lateral sway and stride time variability increase with age [11-13]. Through some of these changes aredue to used to stabilize or posture compensatory, while others are dysfunctional and correlate with the risk of accidental falls [14]. The observed deterioration has been attributed to a variety of causal factors, notably to cognitive decline; indeed, the critical role of cognition is supported by the fact that age-related gait changes are more pronounced in people with cognitive impairment [15-16] and that they are accentuated under dual-task conditions [17-18].
In the previous study, the magnitude of coherence of same and/ or synergy muscle decreased during position holding compared to movement of finger extensor muscle [19]. Decline of especially 15-35 Hz band of coherence of muscle reflects the efferent phenomenon of propagated to the peripheral through the corticospinal tract (CST) by rhythmic activity in the motor cortex [19-21]. Walking performance such as walking speed and stride, have been performed at such a low cognitive function by elderly people [22].
However, it was not clear that the decrease of walking performance during dual task walking in healthy subject, whether affected by decline of activation of CST. In addition, if decrease of coherence of muscle was occurred during dual task walking, it was thought that the dual task was affected cognitive function in upper central and cerebral cortex during walking.
The purpose of this study was to investigate the participation of activation of CST during dual task walking by EMG coherence analysis in healthy adults.
2. Methods
Participates were 6 adults (3 males and 3 females, age 24.0 ± 6.1, height 1.66m ± 0.12). The subjects were healthy and recreationally active with no history of serious lower extremity or, specifically, joint injury. They were fully informed about the risk of experiment and signed an informed consent document before the experiment.
Participates were wearing easy to move clothes and shoes of the usual. They walked on a smooth 10-m horizontal walkaway.The subjects were performed two types walking; normal walking, and dual-task walking. Dual-task walking was achieved calculation task of subtracting serial 7s from 700 aloud during walking. Two gait experiments were performed sequentially.
The walking performances were recorded using a video camera from the right side. The movies were shooting in 60 frames per second. The stride and cadence of steps, walking speed to walk 3 m to the mid-point of the walkaway were measured from video movies, and gait speed was expressed in meters per second (Figure 1).
Bipolar surface electrodes (diameter 10 mm, center-to-center distance 20 mm) were placed over the proximal and distal of tibial anterior (TAd and TAp), medial gastrocnemius (GM), and soleus (Sol) muscles of right leg. The longitudinal axes of the electrodes were parallel to the assumed direction of the underlying muscle fibers. Before the electrode placement, the skin surface was lightly abraded, and cleansed with alcohol to reduce the skin-electrode interface impedance to below 10 kΩ for each subject. Electromyography (EMG) signals were amplified, band-passed filtered between 10 and500 Hz, digitized at 500 Hz and stored in the computer system (EMG master, Mediarea support business union) (Figure 2). Integrated of the EMG (iEMG) signals of TAd, TAp, GM, and Sol were calculated for averaged 1s at the 1 walking cycle phase by normal and dual task walking. Coherence of the EMG signals was calculated for each extensor and flexor muscles using by KyPlot 5.0 (KyensLab Inc.). EMG signals of stored computer system were at first rectified. The rectified EMG signals data were Fast Fourier-transformed, given a resolution of 0.49 Hz. The coherence of each extensor and flexor muscles were calculated by cross-spectra [19,20]. The coherence was calculated frequency and power-spectra were set to be output at the same size by rate of 512 points.The final spectral estimate was obtained Hanning refers to a running smoothing procedure using the weights 1/4, 1/2, 1/4 [19]. The obtained of each waveform by the individual were averaged, and it was compared for each frequency. Analyses of frequency band were 0 to 100 Hz each about 0.5 Hz [23].
Values are given as means ± standard deviation (SD). Because variations among individuals were large, the iEMG amplitudes were normalized to the values of the normal walking condition and compared. The each frequency of coherence of muscle was compared with normal and dual task walking. The walking performance, iEMG, and EMG coherence of muscle were compared to normal walking and dual task walking. Test of iEMG for discrepancy of each normalized mean value from 100% was conducted by performing a t-test for the mean. Statistical significance was set at the 5% level.
3. Results
3.1 Walking performance
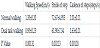
The results of walking performance are summarized in Table 1. The walking speed was decreased about 30 percent in dual task walking compared with normal walking (p<0.01). The stride of steps of dual task walking was decreased about 15 percent of normal walking (p<0.01). Also, the cadence of steps was decreased about 20 percent in dual task walking (p<0.01).
3.2 EMG activity
The values of iEMG of GM and Sol in flexor muscles were not significantly changed (p=0.23, p=0.41, respectively). No significant changed of dual task walking on iEMG of TAd in extensor muscle was observed (p=0.55). The values of the iEMG of TAp of dual task walking were lower compared to normal walking (p<0.05, Figure 3).
3.3 EMG coherence
EMG coherence of each band of dual task walking was not significantly changedeach extensor and flexor musclescompared with normal walking (Figure 4).
4. Discussion
In this study, we considered the walking performance and change of EMG activity of lower limbduring normal walking and dual task walking. As results, it was cleared that the reduction of walking speed,stride, cadence, and iEMG of proximal position of extensor muscle. Although, coherence of extensorand flexor muscles during dual task walking was not significantly changed.
In previous study of walk performance during dual task, it was showed the decreased of walk performance compered with normal walking [4]. Similarly, decrease of walking performance compared to walking speed and stride the normal walkingwas observed by this study.
However, the study of consideration to coherence of same and/ or synergy muscles on lower limb during walking was not found. In this study, coherence of both flexor ad extensor muscles was not changed in dual task walking. Especially, significantly change of beta band widths of 15-35 Hz werenot obtained. Kakuda et al suggested that the coherence of muscle of extensor carpi radialis while subjects performed slow wrist extension and flexion movements was decreased by dual task [19]. In addition, reduction in the coherence of 15- 35Hz bands was to reflect the phenomenon of efferent that rhythmic activity is transmitted to the peripheral through the CST in the motor cortex [19-21]. In this study, decrease of coherence of beta bands was not observed. Previous study was observed significant difference of coherence in such extensor carpi radialis brevis muscle [19], but in this study was measured of coherence intibial anterior muscle. It was suggested that the difference of such measurement points were involved in the difference of coherence of muscle. The activation of CST during walking was enhanced in start and end of walking and avoids obstacles. In start and end of walking, the command was transited by order to midbrain locomotor region (MLR), medullary reticular tract, and central pattern generator (CPG) of the spinal cord [24,25], and the steady state walking was almost used to CPG of the spinal cord. These were suggestedthat the no change of activation of CST in steady state walking during dual task was observed. And no change of muscle coherence during dual task condition was considered to factor of uninvolved CST.
Reduction of iEMG of TAp during dual task walking was only observed. The iEMG activity of TAd, GM and Slo were not changed in dual task walking compared to normal walking. If decreased of iEMG activity was occured, it was thougt that the muscle coherence should be change in extensor muscle. However, no significant difference to the coherence of proximal and distal of extensor was observed. From this, it was suggested that a factor except the output from CST participated in the decrease of the walking function. It was in particular thought that a change of the output to the activity at the spinal cord level influenced walking performance.
In dual task walking, muscle coherence of extensor and flexors has not resulted in significant difference compared to normal walking.
In a survey of the cognitive function of elderly, such as TMT-A, cognitive function test is performed [26]. Additionally, reduction of such as the walking speed and stride during dual task walking has been reported [2-4]. However, it is not only necessary to consider the neural factor but also the walking ability for to examine the decline of cognitive function. It was suggested that the change of walking performance during dual task condition might not be due to decrease of input from CST to motoneurons of distal extensor muscle. It must be shown that the reduction of walking ability during dual task walking was except for the change of central command. Although, method of this present study is not thought to be reveal a part of the change of central command by coherence of muscle in healthy adults. However, this measurement method may be due to consider being one of the ways to investigate the decline of cognitive function in the elderly people.
5. Conclusion
Decline of cognitive was increased risk of fall, and was observed decrease of walking speed andstride length. In healthy subjects, walking performance during dual task was decreased, might be decreased cognitive functionby calculate of numbers.In addition, coherence of extensor and flexor muscles was not changed during dual task walking. MCI and elderly adults consider the measurement method of muscle coherence to effective investigate the decrease of cognitive function. However, in healthy young adults, evaluate of coherence of muscle were not necessary to find by decline of cognitive function.
Competing Interests
The authors declare that they have no competing interests.