1. Introduction
Reports of hypopituitarism resulting from traumatic brain injury (TBI) have been increasing among children and adults over the past decades. In these reports, TBI-related hypopituitarism was found to manifest as either an isolated growth hormone (GH) deficiency or combined hormone deficiency [1-7]. With regard to classical TBI among children, a complicated delivery such as a breech delivery may be a potential cause of hypopituitarism, and GH and adrenocorticotropic hormone (ACTH) deficiencies reportedly progress with age in these so-called ‘classical’ cases [8]. Moreover, cases with an invisible pituitary stalk and ectopic posterior pituitary bright spot on magnetic resonance imaging (MRI) have been reported [9].
To date, four prospective studies on TBI-related hypopituitarism in pediatric cases without perina-tal problems have been published [10-13]; however, the study durations were generally only about one year. Further, although there are at least seven crosssectional studies [14-20] with a comparatively long observational period of up to several years after the TBI episode, the long-term clinical course of postnatal TBI-related hypopituitarism from childhood to adulthood remains to be clarified.
We here present a case involving a 31-year-old Japanese male with hypopituitarism caused by TBI at the age of 5 months. The purpose of this report was to analyze the clinical and endocrinological data on this patient over a period of more than 20 years. As a result, we speculate that the secretion of GH, thyroid-stimulating hormone (TSH), gonadotropins and ACTH gradually decreased in this patient.
2. Case Report
The patient was born uneventfully by spontaneous cephalic delivery at 40 weeks of gestation. Apgar scores at 1 min and 5 min were 10. At birth, his height and weight were 53.0 cm (+1.9 SD) and 3.5 kg (+1.1 SD), respectively. He had no hypoglycemia during the neonatal period or infancy. Micropenis was not observed at the neonatal or infant health check-ups. At age 5 months, he was involved in a motorcycle accident and suffered severe head trauma, including a brain contusion, subdural hemorrhage, fracture of the skull base and cerebral edema, which caused loss of consciousness. He consequently underwent emergency surgery for removal of a hematoma and ventriculoatrial shunt placement at his local hospital. He remained unconscious for the following three days. Seizures developed three times on the fourth day postoperatively, at which time carbamazepine was successfully started. After these episodes, he had normal physical and mental development until the age of 3 years, when the ventriculoatrial shunt was removed. Retrospectively, a decrease in height velocity, the initial symptom, began around the age of 3 years.
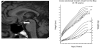
At the age of 7, he was brought to our hospital for retarded growth (Figure 1). His height was 107.4 cm (-3.4 SD) and his weight was 19.3 kg (-1.4 SD). At this time, a micropenis (stretched length: 30 mm, -3.0 SD) was observed. Normal mental development was again documented, with no remarkable medical history other than that described above. He had no polyuria. Endocrinological tests yielded the following results: thyroid-stimulating hormone (TSH) 1.38 μU/ mL, thyroxine 3.91 μg/dL, luteinizing hormone (LH) <0.1 mIU/mL, follicle-stimulating hormone (FSH) <0.1 mIU/mL, insulin-like growth factor-1 52 ng/mL (reference, 63-247 ng/mL) [21], GH response to arginine and insulin 1.8 ng/mL and 2.0 ng/mL, respectively, and cortisol peak to insulin 12.53 μg/dL (Table 1). Based on these findings, GH and TSH deficiencies were diagnosed and treated with GH and l-thyroxine, respectively. GH deficiency was finally proved under euthyroidism at the age of 20 years. Gonadotropin deficiency was suspected but not proved since he was only seven years old. The size of the anterior pituitary gland was small, suggesting hypoplasia. The pituitary stalk was not visible on MRI, and an ectopic posterior pituitary bright spot was noted (Figure 1). At age 10, the peak cortisol response to insulin was 3.0 μg/dL and a diagnosis of ACTH deficiency was made (Table 1). Consequently, hydrocortisone therapy was initiated. The patient had no history of adrenal crisis before starting this therapy. Testosterone enanthate therapy was needed to induce pubertal development at age 14, when hypogonadotropic hypogonadism was confirmed (LH, FSH, testosterone: all below the detection limit). His testicular volumes were 2 mL bilaterally, and his pubic hair was Tanner stage 1 at that time. After the start of GH injections, his growth rate improved, with the patient achieving a final height of 173.0 cm (+0.4 SD) at the age of 31 years (Figure 1). The heights of his father and mother were 172 cm and 162 cm, respectively. His penile length was 9.0 cm, testicular volumes were 4 ml bilaterally, and the pubic hair was Tanner stage 4 at that time.
3. Discussion
We assumed that the evolution of hypopituitarism in this case resulted from a postnatal TBI episode at the age of 5 months. In the present case, the traumatic brain injury included a brain contusion, subdural hemorrhage, fracture of the skull base and cerebral edema, which was considered severe enough to induce hypopituitarism [4]. In previous reports, TBI-related hypopituitarism resulted from severe TBI complications such as vascular thrombosis, infarction, and hemorrhage of the hypothalamic pituitary gland [22-24]. Basal skull fractures may cause direct damage to the hypothalamic-pituitary gland or may indicate a greater contrecoup component of the TBI, consequently resulting in a risk of pituitary stalk disruption [15]. The anterior pituitary is supplied solely by the hypophyseal portal vessels via the long portal vessels, which arise in and run down the free part of the pituitary stalk, having virtually no arterial supply. Thus, extensive necrosis develops if the blood supply is interrupted.
The patient developed hypothalamic hypothyroidism in a functional sense. In TRH stimulation test, the peak value of TSH was 20.53 μIU/mL in 90 min (Table 1), suggesting the pattern of hypothalamic hypothyroidism. Other hypothalamic functions such as posterior pituitary hormone levels, food intake, thirst, and temperature control were intact. There were no abnormal findings of the hypothalamus on MRI. In the present case, we consider that TBI-related permanent hypopituitarism could serve as a model for the evolution of hypopituitarism due to decreased stimuli from the hypothalamus, especially when the patients show the typical MRI findings described above [7,8]. Indeed, mutation of the gene for Ghrhr has been identified in the mouse and results in growth failure and decreased number of somatotrrophs. In this model, the fetal somatotrophs mass is normal, and hypoplasia of the somatotrophs is evident only postnatally [25]. Furthermore, in patients with the mutation, pituitary hypoplasia has been reported postnatally [26]. Therefore, we hypothesize that the MRI findings could be interpreted as reflecting a functional disconnection between the hypothalamus and pituitary [8].
Hypopituitarism progressed with increasing age after the TBI episode in this patient. He experienced uneventful prenatal and perinatal periods and his height velocity began to decrease around the age of 3 years, possibly reflecting the evolution of GH deficiency. Neonatal screening levels of TSH and free T4 were normal. The absence of mental retardation at the age of seven years, when the treatment of hypothyroidism was started, may be consistent with the progression of TSH deficiency.
The ACTH peak level of insulin tests decreased with age. We assumed that ACTH gradually decreased. ACTH deficiency was proven at age 10 because of the low peak levels of ACTH and cortisol (Table 1). We previously reported that the serum cortisol levels tended to be high during stressful episodes in an infant with ACTH deficiency and a coexisting hypothyroid state [27]. In the present patient, the peak cortisol response on an insulin tolerance test at age 7 was obtained before starting thyroxine; thus, the cortisol peak level of insulin test was fallacious. However, he had never had episodes of adrenal crisis before starting hydrocortisone.
He had no micropenis at birth. Progression of gonadotropin deficiency was suspected at age 7 based on his micropenis and the low levels of LH and FSH, but verification was impossible during the pre-puberty stage. Progressing gonadotropin deficiency was verified at age 14 years.
Accordingly, taken together, it is reasonable to infer that GH, and possibly other pituitary hormones such as TSH, ACTH, and gonadotropins, gradually decreased as the patient matured, and previous studies have reported a possibility of this kind of progression after pediatric TBI, leading to permanent hypopituitarism (reviewed in 4).
We consider that ACTH deficiency developed later than GH deficiency in this case, because the growth velocity declined around the age of three years as in Figure 1. Since adrenal sufficiency can be fetal, a possibility of ACTH deficiency should be examined in the follow-up of patients with TBI who have retarded growth. However, the order of deficiency of anterior pituitary hormones has not been extensively described so far in hypopituitarism resulting from TBI. As mentioned above, the follow-up periods after the TBI episode in the four prospective studies of pediatric TBI-related hypopituitarism of around a year are insufficient to analyze the order of the pituitary hormone deficiencies [10-13]. Late onset of ACTH deficiency has also been reported in patients showing an invisible pituitary stalk on MRI, including classical hypopituitarism patients delivered in the breech position [9].
It is reasonable to speculate that the MRI findings described above can be encountered in at least some patients with TBI-related longterm permanent hypopituitarism. The MRI findings includes a lack of visible pituitary stalk and no normal posterior lobe hypersignal in the sella turcica plus a hyperintense nodule in the region of the infundibular recess of the third ventricle, or ectopic posterior lobe. However, the long-term MRI findings of TBI-related hypopituitarism have not been discussed in most previous reports (reviewed in 4). Brain MRI in an adult case with permanent hypopituitarism showed an atrophied pituitary gland [28].
Ectopic posterior lobe could be observed in patients with genetic mutations in OTX2, SOX3, HESX1, and LXH4 [29]. However, we did not find any mutations in the previously reported genes for hypopituitarism (Kazusa Laboratory, Japan; data not shown). This negative finding does not prove but is consistent with the speculation that ectopic posterior pituitary lobe can also be observed in hypopituitarism patients after brain trauma as was illustrated before [7]. Ectopic posterior pituitary lobe is supposed to show AVP storage in the spot, presumably reflecting the functional interruption of the pituitary stalk [29].
4. Conclusion
In conclusion, hypopituitarism progressed with increasing age after the TBI episode in this pa-tient. Given the possibility of the evolution of TBI-related hypopituitarism in children, we should periodically examine the function of the anterior pituitary hormones when following up patients with TBI. This necessity of the long-term follow up is probably true in adult patients with TBI episodes.
Competing Interests
The authors declare that they have no competing interests.